The Realistic Anatomically Detailed Open-source Spinal Cord Stimulation Model (RADO-SCS) is the most anatomically detailed and open-source spinal cord model for simulating all forms of Spinal Cord Stimulation (SCS), DRG stimulation, other forms of spinal cord modulation such as transpinal Direct Current Stimulation (tsDCS), and other forms of spinal modeling such as biomechanical. RADO-SCS is an open source spinal cord stimulation model designed in Solidworks 2016. Model includes detailed structures of the lower thoracic vertebrae (T10-T12) and the spinal column with an emphasis on spinal tissues, nerves, and vasculature. Layers of meninges protecting the gray and white matter such as epidural space, subdural space, arachnoid matter, CSF, and pia matter are designed in detail. Lissauer’s tract and rootlets carrying nerve fibers away from the spinal cord are also included in anatomical detail. STL and Solidworks files for this open source model, as well as any questions on use, can be requested at https://www.neuralengr.org/spinal-cor...
This work has been conducted in collaboration between Dr. Marom Bikson's and Scott Lempka’s research groups. This model of this video was published as and can be cited as: Khadka, N., Liu, X., Zander, H., Swami, J., Rogers, E., Lempka, S., Bikson, M., 2020. Realistic anatomically detailed open-source spinal cord stimulation (RADO-SCS) model. J. Neural Eng. https://doi.org/10.1088/1741-2552/ab8344
This talk given at BioKorea 2020 explains "High-Definition Transcranial Direct Current Stimulation (HD-tDCS) : Low-power, Targeted, Non-invasive Electroceuticals for CNS diseases".
HD-tDCS is special among neuromodulation approaches in that it 1) can be delivered with a battery powered device, 2) it is non-invasive and very well tolerated, can be fully wearable, 3) can be targeted to anatomical regions including using individual MRI or EEG, 4) and can be functionally targeted since it is sub-threshold. No other brain stimulation technique combines all these features, and this talk explains each one in turn.
Watch the talk here
Download the talk PDF
All references (and more) can be found on the lab publication page here
$3 million NIH grant boosts CCNY minority PhD output
In a massive boost to its development of minority PhD students in biomedical disciplines, The City College of New York is the recipient of a new five-year $2,966,693 training grant from the National Institutes of Health (NIH). Prof. Marom Bikson and Prof. Lucas Parra are among 30 participating faculty,
Full press release here
A prospective trial of intraoperative tissue oxygenation measurement and its association with anastomotic leak rate after Ivor Lewis esophagectomy. J Thorac Dis 2020;12(4):1449-1459
Adusumilli PS, Bikson M, Rizk NP, Rusch VW, Hristov B, Grosser R, Tan KS, ISarkaria IS, Huang J, Molena D, Jones DR, Bains MS.
http://dx.doi.org/10.21037/jtd.2020.02.58
Download the PDF
Brainbox Initiative Webinar: The Targeting Limits of Transcranial Electrical Stimulation. Marom Bikson of the The City College of New York
Hear the talk here
Download PDF slide
Some additional Q&A posted here
This free, interactive session will equip delegates with a knowledge of: Modes of transcranial electrical stimulation including conventional tDCS and High-Definition tDCS. Insights on the mechanisms of tDCS that integrate results from advanced current flow and animal models. Using EEG to guide stimulation (reciprocity). New concepts in non-invasive sub-gyri targeting. Functional targeting and Hebbian neuromodulation. The uses and pitfalls of Anode/Cathode based intervention design. Automated tools for individuated modeling. Biophysical insights into Temporal interference stimulation. This webinar will take place at 14:00 BST on May 18, 2020 and will last for approximately 1 hour with time for questions.
New publication:
Guidelines for TMS/tES Clinical Services and Research through the COVID-19 Pandemic
Read it : online
Bikson M, Hanlon CH, Woods AJ, Gillick BT, Charvet L, Lamm C, Madeo G, Holczer A, Almeida J, Antal A, Ay MR, Baeken C, Blumberger DM, Campanella S, Camprodon J, Christiansen L, Colleen L, Crinion J, Fitzgerald P, Gallimberti L, Ghobadi-Azbari P, Ghodratitoostani I, Grabner R, Hartwigsen G, Hirata A, Kirton A, Knotkova H, Krupitsky E, Marangolo M, Nakamura-Palacios EM, Potok W, Praharaj SK, Ruff CC, Schlaug G, Siebner HR, Stagg CJ, Thielscher A, Wenderoth N, Yuan T, Zhang X, Ekhtiari H. . 2020
Dr. Marom Bikson leads with Dr. Hamed Ekhtiari and international team on experts.
We developed a framework for balancing the importance of NIBS operations with safety considerations, which facilitates the re-establishment of access to NIBS clinical services and research operations during COVID-19.
The present consensus paper provides guidelines and good practices for managing and reopening NIBS clinics and laboratories through the immediate and ongoing stages of COVID-19.
The proposed robust and structured strategy aims to address the current and anticipated future challenges while maintaining scientific rigor and managing risk.
Published in Brain Stimulation:
Transcranial Electrical Stimulation Motor Threshold Can Estimate Individualized tDCS Dosage from Reverse-Calculation Electric-Field Modeling
Kevin A. Caulfield, Bashar W. Badran , William H. DeVries, Philipp M. Summers, Emma Kofmehl, Xingbao Li, Jeffrey J. Borckardt, Marom Bikso,n Mark S. George
Free online
Reverse-calculation electric-field modeling can estimate individualized tDCS doses.
Individualized tDCS doses widely vary (range: 3.75 to 9.74mA to produce 1V/m).
•DCS applied at a uniform 1-2mA dose may underdose some individuals.
Transcranial electrical stimulation (TES) motor thresholds (MTs) correlate with reverse-calculation tDCS doses (R2 = 0.45, p < 0.001).
TES MT or reverse-calculation modeling could become methods to individually dose tDCS and warrant further investigation.
Prof. Marom Bikson among the scientists featured in Freethink article “Treating Depression at Home with a tDCS Headset”
Read it here
New publication: Brain Stimulation Journal VOLUME 13, ISSUE 3, P686-693, MAY 01, 2020
Supervised transcranial direct current stimulation (tDCS) at home: A guide for clinical research and practice Leigh E. Charvet. Michael T. Shaw, Marom Bikson, Adam J. Woods Helena Knotkova
Background: Transcranial direct current stimulation (tDCS) is a method of noninvasive neuromodulation and potential therapeutic tool to improve functioning and relieve symptoms across a range of central and peripheral nervous system conditions. Evidence suggests that the effects of tDCS are cumulative with consecutive daily applications needed to achieve clinically meaningful effects. Therefore, there is growing interest in delivering tDCS away from the clinic or research facility, usually at home. Objective: To provide a comprehensive guide to operationalize safe and responsible use of tDCS in home settings for both investigative and clinical use. Methods: Providing treatment at home can improve access and compliance by decreasing the burden of time and travel for patients and their caregivers, as well as to reach those in remote locations and/or living with more advanced disabilities. Results: To date, methodological approaches for at-home tDCS delivery have varied. After implementing the first basic guidelines for at-home tDCS in clinical trials, this work describes a comprehensive guide for facilitating safe and responsible use of tDCS in home settings enabling access for repeated administration over time. Conclusion: These guidelines provide a reference and standard for practice when employing the use of tDCS outside of the clinic setting.
Niranjan Khadka, Xijie Liu, Hans Zander, Jaiti Swami, Evan Rogers, Scott F Lempka, Marom Bikson. Realistic anatomically detailed open-source spinal cord stimulation (RADO-SCS) model. Journal of Neural Engineering 2020. DOI: 10.1088/1741-2552/ab8344
Download PDF published in Journal of Neural Enginnering — DOI
Abstract
Objective:
Computational current flow models of spinal cord stimulation (SCS) are widely used in device development, clinical trial design, and patient programming. Proprietary models of varied sophistication have been developed. An open-source model with state-of-the-art precision would serve as a standard for SCS simulation.
Approach:
We developed a sophisticated SCS modeling platform, named Realistic Anatomically Detailed Open-Source Spinal Cord Stimulation (RADO-SCS) model. This platform consists of realistic and detailed spinal cord and ancillary tissues anatomy derived based on prior imaging and cadaveric studies. In our finite element model of the T9-T11 spine levels, we represented the following tissues: vertebrae, intervertebral disc, epidural space, epidural space vasculature, dura mater, dural sac, intraforaminal tissue, cerebrospinal fluid (CSF), whitematter, spinal cord vasculature, Lissauer’s tract, gray matter, dorsal and ventral roots and rootlets, dorsal root ganglion (DRG), sympathetic chain (trunk and ganglion), thoracic aorta and its branching, peripheral vasculature, and soft tissues (thorax). As an exemplary application to illustrate the model workflow, we simulated a bipolar SCS montage and calculated the corresponding activation thresholds for individual axons populating the spinal cord.
Main results:
RADO-SCS provides state-of-the-art precision across 19 tissue compartments. The resulting model calculations of the electric fields generated in the white-matter and gray matter, and the axonal activation thresholds are broadly consistent with prior simulations.
Significance:
The RADO-SCS can be used to simulate any SCS approach with both unprecedented resolution (precision) and transparency (reproducibility). Freely-available online, the RADO-SCS will be updated continuously with version control.
Transcranial Electrical and Magnetic Stimulation (tES and TMS) for Addiction Medicine: A consensus paper on the present state of the science and the road ahead. https://doi.org/10.1016/j.neubiorev.2019.06.007. PDF
Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald W, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noël X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron BJ, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan T-F, van Dongen J, Van Waes V, Venkatasubramanian G, VerdejoGarcía A, Verveer I, Welsh J, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast M-R, Zawertailo L, Zhang X, Cha Y-H, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA (2019). Neuroscience & Biobehavioral Reviews. 2019. 104: 118-140
Abstract: There is growing interest in non-invasive brain stimulation (NIBS) as a novel treatment option for substance-use disorders (SUDs). Recent momentum stems from a foundation of preclinical neuroscience demonstrating links between neural circuits and drug consuming behavior, as well as recent FDA-approval of NIBS treatments for mental health disorders that share overlapping pathology with SUDs. As with any emerging field, enthusiasm must be tempered by reason; lessons learned from the past should be prudently applied to future therapies. Here, an international ensemble of experts provides an overview of the state of transcranial-electrical (tES) and transcranial-magnetic (TMS) stimulation applied in SUDs. This consensus paper provides a systematic literature review on published data – emphasizing the heterogeneity of methods and outcome measures while suggesting strategies to help bridge knowledge gaps. The goal of this effort is to provide the community with guidelines for best practices in tES/TMS SUD research. We hope this will accelerate the speed at which the community translates basic neuroscience into advanced neuromodulation tools for clinical practice in addiction medicine.
NYC Neuromodulation Online 2020 Conference will be a virtual, self-organized and self-managed meeting spanning 3 days. The conference will run continuously from April 20th at 9 AM (ET) to April 22nd 9 PM (ET). Any member of the scientific community can develop and submit a session and registration is free of charge and open to everyone.
All meetings must be conducted via Zoom video-conferencing software. After an organizer submits a session, it goes into the review phase by our Conference Scientific Committee. When the session is approved it is posted on our website and participants can start reserving their seats.
NYC Neuromodulation started as a grass-roots non-profit meeting first held in 2015 at the City College of New York in New York City. In 2017, NYC Neuromodulation was run again at The City College of New York. In 2018, NYC Neuromodulation was run jointly with the North American Neuromodulation Society (NANS) at the Sheraton, New York City, In 2019, NYC Neuromodulation was run jointly with the Neuromodulation: The Science conference in Napa, California. The NYC Neuromodulation Online 2020 conference continues in grass-roots spirit, opening up the conference attendance and organization to the neuromodulation community.
New publication:
Front. Hum. Neurosci., 18 March 2020 | https://doi.org/10.3389/fnhum.2020.00077
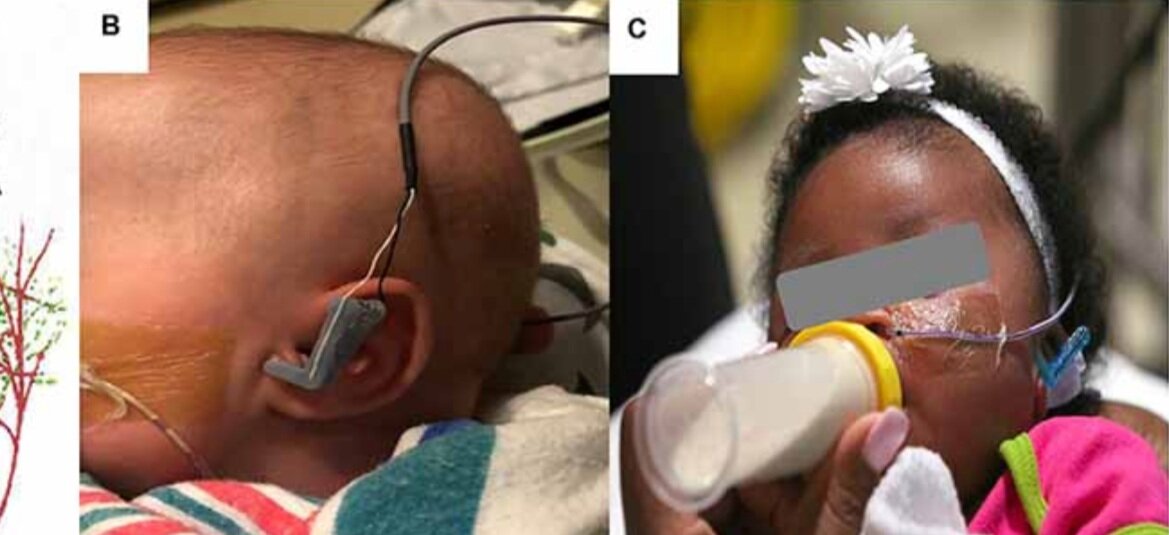
Transcutaneous Auricular Vagus Nerve Stimulation-Paired Rehabilitation for Oromotor Feeding Problems in Newborns: An Open-Label Pilot Study
Bashar W. Badran, Dorothea D. Jenkins, Daniel Cook, Sean Thompson, Morgan Dancy, William H. DeVries, Georgia Mappin, Philipp Summers, Marom Bikson and Mark S. George
Abstract: Neonates born premature or who suffer brain injury at birth often have oral feeding dysfunction and do not meet oral intake requirements needed for discharge. Low oral intake volumes result in extended stays in the hospital (>2 months) and can lead to surgical implant and explant of a gastrostomy tube (G-tube). Prior work suggests pairing vagus nerve stimulation (VNS) with motor activity accelerates functional improvements after stroke, and transcutaneous auricular VNS (taVNS) has emerged as promising noninvasive form of VNS. Pairing taVNS with bottle-feeding rehabilitation may improve oromotor coordination and lead to improved oral intake volumes, ultimately avoiding the need for G-tube placement. We investigated whether taVNS paired with oromotor rehabilitation is tolerable and safe and facilitates motor learning in infants who have failed oral feeding. We enrolled 14 infants [11 premature and 3 hypoxic–ischemic encephalopathy (HIE)] who were slated for G-tube placement in a prospective, open-label study of taVNS-paired rehabilitation to increase feeding volumes. Once-daily taVNS was delivered to the left tragus during bottle feeding for 2 weeks, with optional extension. The primary outcome was attainment of oral feeding volumes and weight gain adequate for discharge without G-tube while also monitoring discomfort and heart rate (HR) as safety outcomes. We observed no adverse events related to stimulation, and stimulation-induced HR reductions were transient and safe and likely confirmed vagal engagement. Eight of 14 participants (57%) achieved adequate feeding volumes for discharge without G-tube (mean treatment length: 16 ± 6 days). We observed significant increases in feeding volume trajectories in responders compared with pre-stimulation (p < 0.05). taVNS-paired feeding rehabilitation appears safe and may improve oral feeding in infants with oromotor dyscoordination, increasing the rate of discharge without G-tube, warranting larger controlled trials.
Held online (following Covid-19 concerns)
Dr. Marom Bikson speaks on “Modeling of Brain & Cranial Nerve Activation”. Download the sides here
Conference and viewing information here
Conference overview: Non-invasive vagus nerve stimulation (VNS) may be administered via a novel, emerging neuromodulatory technique known as transcutaneous auricular vagus nerve stimulation (taVNS). Unlike cervically-implanted VNS, taVNS is an inexpensive and non-surgical alternative to modulate the vagus nerve. taVNS delivers electrical stimulation to the auricular branch of the vagus nerve (ABVN), an easily accessible target that innervates the human ear and is appealing as it allows for rapid translation of basic VNS research. Interest in taVNS has grown over the past decade with many promising neuropsychiatric disease applications such as stroke and depression, as well as enhancing neuroplasticity and reducing inflammation. This safe, inexpensive, and portable neurostimulation modality has major implications in the future treatment of central and peripheral disease. This 2-day conference, organized by recognized taVNS expert Dr. Bashar Badran, is designed to provide lectures by international taVNS experts and hands-on opportunities to learn and practice the fundamentals of noninvasive vagus nerve stimulation.
New publication and technology:
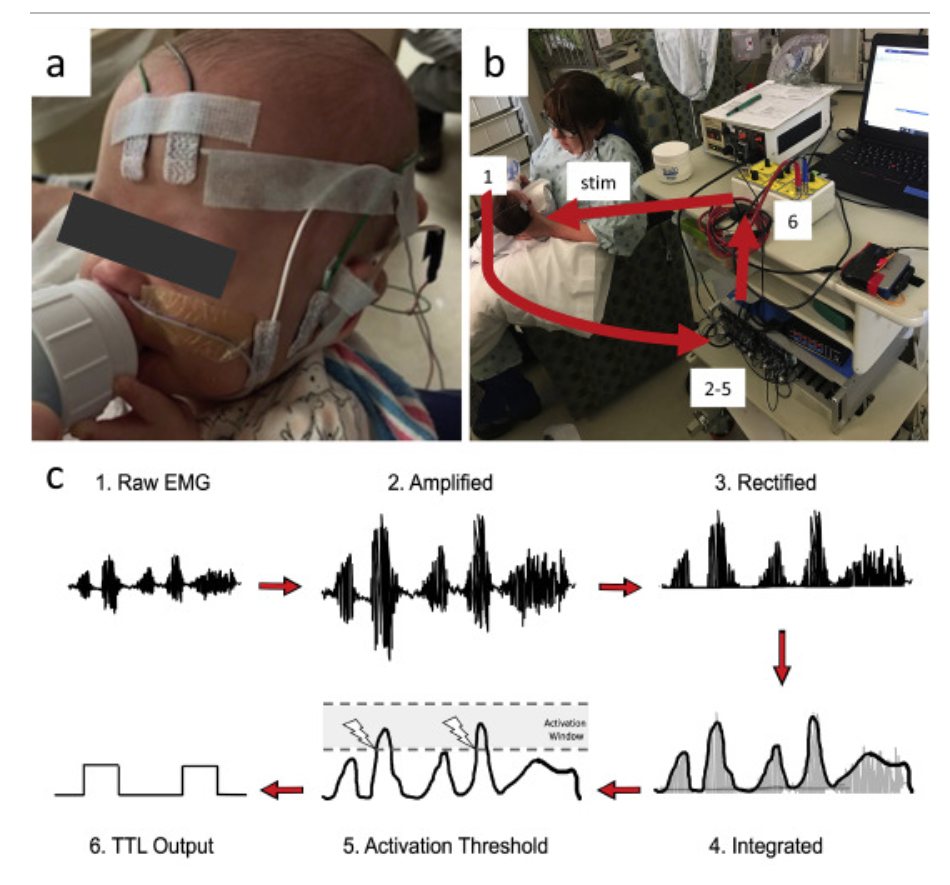
Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation
Daniel N. Cook , Sean Thompson , Sasha Stomberg-Firestein , Marom Bikson, Mark S. George , Dorothea D. Jenkins , Bashar W. Badran
Abstract: Background: Studies have found that pairing vagus nerve stimulation (VNS) with motor activity accelerates cortical reorganization. This synchronous pairing may enhance motor recovery. Objective: To develop and validate a motor-activated auricular vagus nerve stimulation (MAAVNS) system as a potential neurorehabilitation tool. Methods: We created MAAVNS and validated its function as part of an ongoing clinical trial investigating whether taVNS-paired rehabilitation enhances oromotor learning. We compared 3 different MAAVNS EMG electrode configurations in 3 neonates. The active lead was placed over the buccinator muscle. Reference lead placements were orbital, temporal or frontal. Results: The frontal reference lead produced the highest sensitivity (0.87 ± 0.07 (n ¼ 8)) and specificity (0.64 ± 0.13 (n ¼ 8)). Oral sucking reliably triggers MAAVNS stimulation with high confidence. Conclusion: EMG electrodes placed on target orofacial muscles can effectively trigger taVNS stimuli in infants in a closed loop fashion.
Prof. Marom Bikson hosts the first 2020 Jake Zabara Lectureship in NeuroCybernetic Modulation
Wednesday, February 26, 2020, 3:00-4:00 PM, Reception Follows, Location: ASRC Auditorium
Invited 2020 Jake Zabara Lectureship in NeuroCybernetic Modulation speaker: Brian H. Kopell, MD
" The nervous system is not a light switch-VNS, Neuromodulation and Neuroplasticity”
Dr. Brian Kopell is a Professor of Neurosurgery, Neurology, Psychiatry, and Neuroscience and the Director of the Center for Neuromodulation at the Mount Sinai Health System. Dr. Kopell has pioneered the use of neuromodulation technologies including Vagus Nerve Stimulation (VNS) and Deep Brain Stimulation (DBS) for the treatment of neurological and psychiatric disorders. Dr. Kopell will give the first annual Jake Zabara Lectureship in NeuroCybernetic Modulation on the history, current clinical impact, and future development of VNS.
With opening remarks by Dr. Jake Zabara. Dr. Zabara is the inventor of Vagus Nerve Stimulation (VNS) therapy, commercialized for the treatment of Epilepsy and Depression by LivaNova (formally Cyberonics).
Update- Images from Event:
New lab publication: Impact of brain atrophy on tDCS and HD-tDCS current flow: a modeling study in three variants of primary progressive aphasia.
Neurological Sciences https://doi.org/10.1007/s10072-019-04229-z
Gozde Unal, Bronte Ficek, Kimberly Webster, Syed Shahabuddin, Dennis Truong, Benjamin Hampstead, Marom Bikson, Kyrana Tsapkini
Download PDF
Abstract: Background During transcranial direct current stimulation (tDCS), the amount and distribution of current that reaches the brain depends on individual anatomy. Many progressive neurodegenerative diseases are associated with cortical atrophy, but the importance of individual brain atrophy during tDCS in patients with progressive atrophy, including primary progressive aphasia (PPA), remains unclear. Objective In the present study, we addressed the question whether brain anatomy in patients with distinct cortical atrophy patterns would impact brain current intensity and distribution during tDCS over the left IFG. Method We developed state-of-the-art, gyri-precise models of three subjects, each representing a variant of primary progressive aphasia: non-fluent variant PPA (nfvPPA), semantic variant PPA (svPPA), and logopenic variant PPA (lvPPA). We considered two exemplary montages over the left inferior frontal gyrus (IFG): a conventional pad montage (anode over F7, cathode over the right cheek) and a 4 × 1 high-definition tDCS montage. We further considered whether local anatomical features, specifically distance of the cortex to skull, can directly predict local electric field intensity. Results We found that the differences in brain current flow across the three PPA variants fall within the distribution of anatomically typical adults. While clustering of electric fields was often around individual gyri or sulci, the minimal distance from the gyri/sulci to skull was not correlated with electric field intensity. Conclusion Limited to the conditions and assumptions considered here, this argues against a specific need to adjust the tDCS montage for these patients any more than might be considered useful in anatomically typical adults. Therefore, local atrophy does not, in isolation, reliably predict local electric field. Rather, our results are consistent with holistic head anatomy influencing brain current flow, with tDCS producing diffuse and individualized brain current flow patterns and HD-tDCS producing targeted brain current flow across individuals.
Niranjan Khadka, Irene E. Harmsen, Andres M. Lozano, Marom Bikson. Bio-Heat Model of Kilohertz-Frequency Deep Brain Stimulation Increases Brain Tissue Temperature. Neuromodulation 2020. DOI: 10.1111/ner.13120
Download PDF published in Neuromodulation — DOI
Abstract
Objectives
Early clinical trials suggest that deep brain stimulation at kilohertz frequencies (10 kHz‐DBS) may be effective in improving motor symptoms in patients with movement disorders. The 10 kHz‐DBS can deliver significantly more power in tissue compared to conventional frequency DBS, reflecting increased pulse compression (duty cycle). We hypothesize that 10 kHz‐DBS modulates neuronal function through moderate local tissue heating, analogous to kilohertz spinal cord stimulation (10 kHz‐SCS). To establish the role of tissue heating in 10 kHz‐DBS (30 μs, 10 kHz, at intensities of 3‐7 mApeak), a decisive first step is to characterize the range of temperature changes during clinical kHz‐DBS protocols.
Materials and Methods
We developed a high‐resolution magnetic resonance imaging‐derived DBS model incorporating joule‐heat coupled bio‐heat multi‐physics to establish the role of tissue heating. Volume of tissue activated (VTA) under assumptions of activating function (for 130 Hz) or heating (for 10 kHz) based neuromodulation are contrasted.
Results
DBS waveform power (waveform RMS) determined joule heating at the deep brain tissues. Peak heating was supralinearly dependent on stimulation RMS. The 10 kHz‐DBS stimulation with 2.3 to 5.4 mARMS (corresponding to 3 to 7 mApeak) produced 0.10 to 1.38°C heating at the subthalamic nucleus (STN) target under standard tissue parameters. Maximum temperature increases were predicted inside the electrode encapsulation layer (enCAP) with 2.3 to 5.4 mARMS producing 0.13 to 1.87°C under standard tissue parameters. Tissue parameter analysis predicted STN heating was especially sensitive (ranging from 0.44 to 1.35°C at 3.8 mARMS) to decreasing enCAP electrical conductivity and decreasing STN thermal conductivity.
Conclusions
Subject to validation with in vivo measurements, neuromodulation through a heating mechanism of action by 10 kHz‐DBS can indicate novel therapeutic pathways and strategies for dose optimization.
Dr. Marom Bikson co-direct (with Dr. Scott Lempka) the North American Neuromodulation Society (NANS) pre-conference workshop, “Engineering principles of spinal cord stimulation and deep brain stimulation for clinicians,” from 8:00am to 5:00pm on Thursday January 23, 2020 at Caesars Palace in Las Vegas, NV.
The exciting workshop will present engineering principles relevant to Spinal Cord Stimulation (SCS) and Deep Brain Stimulation (DBS). The target audience is clinicians that currently utilize or are interested in incorporating SCS and/or DBS technologies into their clinical practice. The field of neuromodulation is rapidly evolving and several technologies are now clinically available. These systems have diverse lead and stimulator designs. It is not always clear how different designs or waveform parameters affect the neural response and corresponding efficacy of the stimulation. Therefore, the goal of this course is to provide clinicians with an overview of the engineering principles and biophysics relevant to SCS and DBS. This course will also present the current understanding of the physiological effects and mechanisms of action of standard and emerging forms of SCS and DBS. Furthermore, this course will describe safety and regulatory issues that are critical to electrode design and stimulation parameters. At the end of the course, attendees will have a better understanding of the physiological and technical factors that determine the neural response to SCS and DBS. The ultimate goal of this course is to provide attendees with knowledge that will aid in their clinical implementation of SCS and DBS
Dr. Bikson provides two lectures in the course:
08:10 – 08:50 am: Neurostimulation fundamentals – waveform basics, membrane polarization, action potential threshold, region of activation. Download the lecture slides
02:00 – 02:30 pm: Sub-threshold mechanisms and heating in Spinal Cord Stimulation (SCS) and Deep Brain Stimulation (DBS) Download the lecture slides