Presented on on Jan 15th, 2022. Here are the slides
Marom Bikson co-directs with Scott Lempka the”Engineering principles of DBS and SCS in clinical practice: General introduction and emerging concepts” pre-conference course on Jan 13, 2022
CCNY Neural Engineering is at the North American Neuromodulation Society (NANS) 2022 meerting:
Dr. Bikson also lectures at the Jan 13, 2022 pre-conference course on “Neurostimulation fundamentals: Dose, current flow, and neural activation.” Download slides
Jan 14, 2022 Dr. Bikson lectures on “Spinal Cord Stimulation (SCS): Subthreshold Mechanisms.” in the Engineering Principles in Neurostimulation: Emerging Concepts session 1/14/2022, 10:30 am - 12:00 pm Download slides
“Zapping the Brain and Nerves Could Treat Long COVID Pilot studies test electrical treatments for the still-mysterious malady” by ELIZA STRICKLAND In IEEE Spectrum. Read the article here
The Bikson lab’s work with New York University (NYU) Langone, Federal University of Paraiba (Brazil), and Medical University of South Carolina were featured.
“Marom Bikson notes that both the research field and the industry of neurostimulation is just getting started. “We don’t have Pfizers of neuromodulation,” he says, “but you can only imagine what would happen if it shows an effect on long COVID.” It could lead to millions of people having stimulators in their homes, he suggests, which could open other doors. “Once you start stimulating for long COVID, you can start stimulating for other things like depression,” he says. But he says it’s crucial to proceed cautiously and not make unsupported claims for neurostimulation’s powers. “Otherwise,” he says, “it could have the opposite effect.”
Pictured are Edson Meneses, Gozde Unal, Nigel Gebodh, and Marom Bikson
Dr. Marom Bikson will speak at the American Association for the Advancement of Science (AAAS) annual meeting.
PDF of slides: download
Transcranial Electrical Stimulation (tES)
Niranjan Khadka, Marom Bikson
NeuroTechX (2021) |The NeuroTech Primer: A Beginner’s Guide to Everything Neurotechnology | ASIN: B09CKP1D66 | ISBN: 979-8454254056| Pp: 109-125 |
Abstract:
Transcranial electrical stimulation (tES) devices apply electrical waveforms through electrodes placed on the scalp to modulate brain function. Various types of tES devices are used for a wide range of indications spanning neurological and psychiatric disorders, blood-brain barrier polarization, neurorehabilitation after injury, and altering cognition in healthy adults. All tES devices share certain common features including a waveform generator (typically a current controlled source), electrodes that are either fully disposable or include a disposable electrolyte, and an adhesive to position the electrodes on the scalp. Various tES subclasses are named based on dose. For example, Electroconvulsive therapy (ECT) is a special class of tES applying high stimulation intensity. tES “dose” is defined by the size and position of electrodes, and waveform including the pattern, duration, and intensity of the current.
The 4th International Brain Stimulation Conference on Dec 6-9. 2021 in Charleston. Conference info
The CCNY Neural Engineering lab will be making multiple presentations:
Talks
tDCS news: COVID-19 and PASC treatment, Neurovascular-modulation, and Games
Marom Bikson Slides PDF
Computational model of electroconvulsive therapy considering electric field dependent skin conductivity
Gozde Unal , Jaiti Swami, Carliza Canela, Samantha Cohen, Niranjan Khadka, Mohammad Rad1, Baron Short, Miklos Argyelan, Harold Sackeim, Marom Bikson
History and recent advancements and changes in computational modeling methods for transcranial electrical stimulation slides
Marom Bikson
Poster Presentations
A large open source neuromodulation dataset of concurrent EEG, ECG, behavior, and transcranial electrical stimulation
Nigel Gebodh, Zeinab Esmaeilpour, Abhishek Datta, Marom Bikson
P1.106 | Full Abstract >>
A novel approach to closed-loop neuromodulation with machine learning
Nigel Gebodh, Marom Bikson
P2.106 | Full Abstract >>
Neurocapillary-modulation
Niranjan Khadka, Marom Bikson
P3.006 | Full Abstract >>
Computational model of electroconvulsive therapy considering electric field dependent skin conductivity
Gozde Unal, Jaiti Swami, Carliza Canela,...Miklos Argyelan, Harold Sackeim, Marom Bikson
P3.102 | Full Abstract >>
Prof. Marom Bikson lectures at the online XIII International Symposium on Neuromodulation on Nov 21, 2022 on the topic of “Transcranial direct current stimulation changes brain vasculature (and Non-invasive neuromodulation in the post COVID-19 world)”.
Conference program (Nov 21-24, 2022)
Downloads slides PDF
New paper in Brain Stimulation
Weak DCS causes a relatively strong cumulative boost of synaptic plasticity with spaced learning
Mahim Sharma, Forouzan Farahani, Marom Bikson, & Lucas C. Parra
Brain Stimulation | (2021) 15(1):57–62 | https://doi.org/10.1016/j.brs.2021.10.552
Download PDF
Abstract: Background: Electric fields generated during direct current stimulation (DCS) are known to modulate activity-dependent synaptic plasticity in-vitro. This provides a mechanistic explanation for the lasting behavioral effects observed with transcranial direct current stimulation (tDCS) in human learning experiments. However, previous in-vitro synaptic plasticity experiments show relatively small effects despite using strong fields compared to what is expected with conventional tDCS in humans (20 V/m vs. 1 V/m). There is therefore a need to improve the effectiveness of tDCS at realistic field intensities. Here we leverage the observation that effects of learning are known to accumulate over multiple bouts of learning, known as spaced learning.
Hypothesis: We propose that effects of DCS on synaptic long-term potentiation (LTP) accumulate over time in a spaced learning paradigm, thus revealing effects at more realistic field intensities.
Methods: We leverage a standard model for spaced learning by inducing LTP with repeated bouts of theta burst stimulation (TBS) in hippocampal slice preparations. We studied the cumulative effects of DCS paired with TBS at various intensities applied during the induction of LTP in the CA1 region of rat hippocampal slices. Results: As predicted, DCS applied during repeated bouts of theta burst stimulation (TBS) resulted in an increase of LTP. This spaced learning effect is saturated quickly with strong TBS protocols and stronger fields. In contrast, weaker TBS and the weakest electric fields of 2.5 V/m resulted in the strongest relative efficacies (12% boost in LTP per 1 V/m applied).
Conclusions: Weak DCS causes a relatively strong cumulative effect of spaced learning on synaptic plasticity. Saturation may have masked stronger effects sizes in previous in-vitro studies. Relative effect sizes of DCS are now closer in line with human tDCS experiments.
Title: Technology and science of of transcranial Direct Current Stimulation (tDCS) can boost brain function and capacity for plasticity
Abstract : Transcranial direct current stimulation (tDCS) is a non-invasive wearable technique where weak direct current is applied to the brain. This presentation explains the technological basics of tDCS and its understood mechanisms of action, along with how tDCS can be customized to diverse applications. Topics covered:
1) Basics of tDCS dosing including electrode placement, conventional and HD electrodes
3) Customizing electrode placement for subjects based on individual anatomical MRI or functional imaging
4) "Functional targeting" a mechanism to boost the efficacy of cognitive and behavioral therapies
5) A new concept of "neurovascular modulation", where tDCS direct activates vascular function blood flow and BBB transport
Download slide: PDF
New paper in Scientific Data
Dataset of concurrent EEG, ECG, and behavior with multiple doses of transcranial electrical stimulation
Nigel Gebodh, Zeinab Esmaeilpour, Abhishek Datta & Marom Bikson
Nature Scientific Data | (2021) 8, 274 | https://doi.org/10.1038/s41597-021-01046-y
Download PDF
Abstract: We present a dataset combining human-participant high-density electroencephalography (EEG) with physiological and continuous behavioral metrics during transcranial electrical stimulation (tES). Data include within participant application of nine High-Definition tES (HD-tES) types, targeting three cortical regions (frontal, motor, parietal) with three stimulation waveforms (DC, 5 Hz, 30 Hz); more than 783 total stimulation trials over 62 sessions with EEG, physiological (ECG, EOG), and continuous behavioral vigilance/alertness metrics. Experiment 1 and 2 consisted of participants performing a continuous vigilance/alertness task over three 70-minute and two 70.5-minute sessions, respectively. Demographic data were collected, as well as self-reported wellness questionnaires before and after each session. Participants received all 9 stimulation types in Experiment 1, with each session including three stimulation types, with 4 trials per type. Participants received two stimulation types in Experiment 2, with 20 trials of a given stimulation type per session. Within-participant reliability was tested by repeating select sessions. This unique dataset supports a range of hypothesis testing including interactions of tDCS/tACS location and frequency, brain-state, physiology, fatigue, and cognitive performance.
Transcranial electrical stimulation devices
Dennis Q. Truong, Niranjan Khadka, Angel V. Peterchev, and Marom Bikson
Oxford University Press | (2021) | The Oxford Handbook of Transcranial Stimulation, Second Edition 2 2-55 | 10.1093/oxfordhb/9780198832256.013.2
Download PDF
Abstract:
Transcranial electrical stimulation (tES) devices apply electrical waveforms through electrodes placed on the scalp to modulate brain function. This chapter describes the principles, types, and components of tES devices as well as practical considerations for their use. All tES devices include a waveform generator, electrodes, and an adhesive or headgear to position the electrodes. tES dose is defined by the size and position of electrodes, and the waveform, duration, and intensity of the current. Many sub-classes of tES are named based on dose. This chapter focuses on low intensity tES, which includes transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial pulsed current stimulation (tPCS). tES electrode types are reviewed, including electrolyte-soaked sponge, adhesive hydrogel, high-definition, hand-held solid metal, free paste on electrode, and dry. Computational models support device design and individual targeting. The tolerability of tES is protocol specific, and medical grade devices minimize risk.
New paper in Frontiers in Cell and Developmental Biology
Direct Current Stimulation Disrupts Endothelial Glycocalyx and Tight Junctions of the Blood-Brain Barrier in vitro
Yifan Xia, Yunfei Li, Wasem Khalid, Marom Bikson & Bingmei M. Fu
Frontiers in Cell and Developmental Biology | (2021) 9 | https://doi.org/10.3389/fcell.2021.731028
Download PDF
Abstract: Transcranial direct current stimulation (tDCS) is a non-invasive physical therapy to treat many psychiatric disorders and to enhance memory and cognition in healthy individuals. Our recent studies showed that tDCS with the proper dosage and duration can transiently enhance the permeability (P) of the blood-brain barrier (BBB) in rat brain to various sized solutes. Based on the in vivo permeability data, a transport model for the paracellular pathway of the BBB also predicted that tDCS can transiently disrupt the endothelial glycocalyx (EG) and the tight junction between endothelial cells. To confirm these predictions and to investigate the structural mechanisms by which tDCS modulates P of the BBB, we directly quantified the EG and tight junctions of in vitro BBB models after DCS treatment. Human cerebral microvascular endothelial cells (hCMECs) and mouse brain microvascular endothelial cells (bEnd3) were cultured on the Transwell filter with 3 μm pores to generate in vitro BBBs. After confluence, 0.1–1 mA/cm2 DCS was applied for 5 and 10 min. TEER and P to dextran-70k of the in vitro BBB were measured, HS (heparan sulfate) and hyaluronic acid (HA) of EG was immuno-stained and quantified, as well as the tight junction ZO-1. We found disrupted EG and ZO-1 when P to dextran-70k was increased and TEER was decreased by the DCS. To further investigate the cellular signaling mechanism of DCS on the BBB permeability, we pretreated the in vitro BBB with a nitric oxide synthase (NOS) inhibitor, L-NMMA. L-NMMA diminished the effect of DCS on the BBB permeability by protecting the EG and reinforcing tight junctions. These in vitro results conform to the in vivo observations and confirm the model prediction that DCS can disrupt the EG and tight junction of the BBB. Nevertheless, the in vivo effects of DCS are transient which backup its safety in the clinical application. In conclusion, our current study directly elucidates the structural and signaling mechanisms by which DCS modulates the BBB permeability.
New Publication in Brain Stimulation
High-resolution computational modeling of the current flow in the outer ear during transcutaneous auricular Vagus Nerve Stimulation (taVNS)
Erica Kreisberg, Zeinab Esmaeilpoura, Devin Adair, Niranjan Khadka, Abhishek Datta, Bashar W. Badran, Douglas Bremner, Marom Bikson
Brain Stimulation | (2021) 14 1419- 1430 | https://doi.org/10.1016/j.brs.2021.09.001
Download PDF
Abstract:
Background
Transcutaneous auricular Vagus Nerve Stimulation (taVNS) applies low-intensity electrical current to the ear with the intention of activating the auricular branch of the Vagus nerve. The sensitivity and selectivity of stimulation applied to the ear depends on current flow pattern produced by a given electrode montage (size and placement).
Objective
We compare different electrodes designs for taVNS considering both the predicted peak electric fields (sensitivity) and their spatial distribution (selectivity).
Methods
Based on optimized high-resolution (0.47 mm) T1 and T2 weighted MRI, we developed an anatomical model of the left ear and the surrounding head tissues including brain, CSF/meninges, skull, muscle, blood vessels, fat, cartilage, and skin. The ear was further segmented into 6 regions of interest (ROI) based on various nerve densities: cavum concha, cymba concha, crus of helix, tragus, antitragus, and earlobe. A range of taVNS electrode montages were reproduced spanning varied electrodes sizes and placements over the tragus, cymba concha, earlobe, cavum concha, and crus of helix. Electric field across the ear (from superficial skin to cartilage) for each montage at 1 mA or 2 mA taVNS, assuming an activation threshold of 6.15 V/m, 12.3 V/m or 24.6 V/m was predicted using a Finite element method (FEM). Finally, considering every ROI, we calculated the sensitivity and selectivity of each montage.
Results
Current flow patterns through the ear were highly specific to the electrode montage. Electric field was maximal at the ear regions directly under the electrodes, and for a given total current, increases with decreasing electrode size. Depending on the applied current and nerves threshold, activation may also occur in the regions between multiple anterior surface electrodes. Each considered montage was selective for one or two regions of interest. For example, electrodes across the tragus restricted significant electric field to the tragus. Stimulation across the earlobe restricted significant electric field to the earlobe and the antitragus. Because of this relative selectivity, use of control ear montages in experimental studies, support testing of targeting. Relative targeting was robust across assumptions of activation threshold and tissue properties.
Discussion
Computational models provide additional insight on how details in electrode shape and placement impact sensitivity (how much current is needed) and selectivity (spatial distribution), thereby supporting analysis of existing approaches and optimization of new devices. Our result suggest taVNS current patterns and relative target are robust across individuals, though (variance in) axon morphology was not represented.
Segmented anatomy of outer ear, the region of interests (ROIs) of ABVN, and simulated taVNS montages. A1-A5, B1-B3 represent segmentation of outer ear skin, outer ear cartilage, fat, muscle, blood vessels, CSF, brain/grey-matter, as well as color coded ROIs (B4). C1-C10 indicate montages simulated.
Dr. Marom Bikson will participate in the 2021 Neuroergonomics Conference online.
Dr. Bikson will speak on “Can Neuromodulation Make Us Better: Changing Brain Activity with Wearable Brain Stimulation Devices” on Sept 15th. Download slides PDF
Dr. Bikson will co-direct a workshop Introduction to practical methods in low-intensity transcranial Electrical Stimulation, Sep 12th. Slides from the tES Workshop PDF
New publication in Neuroimage: Reports
Investigating the brain regions involved in tDCS-Enhanced category learning using finite element modeling
Aaron P. Jones, Monica Goncalves-Garcia, Benjamin Gibson, Michael C.S. Trumbo, Brian A. Coffman, Bradley Robert, Hope A. Gill, Teagan Mullins, Michael A. Hunter, Charles S.H. Robinson, Angela Combs, Niranjan Khadka, Marom Bikson, Vincent P. Clark
Neuroimage Reports | (2021) 4(1):100048 | https://doi.org/10.1016/j.ynirp.2021.100048
Download PDF
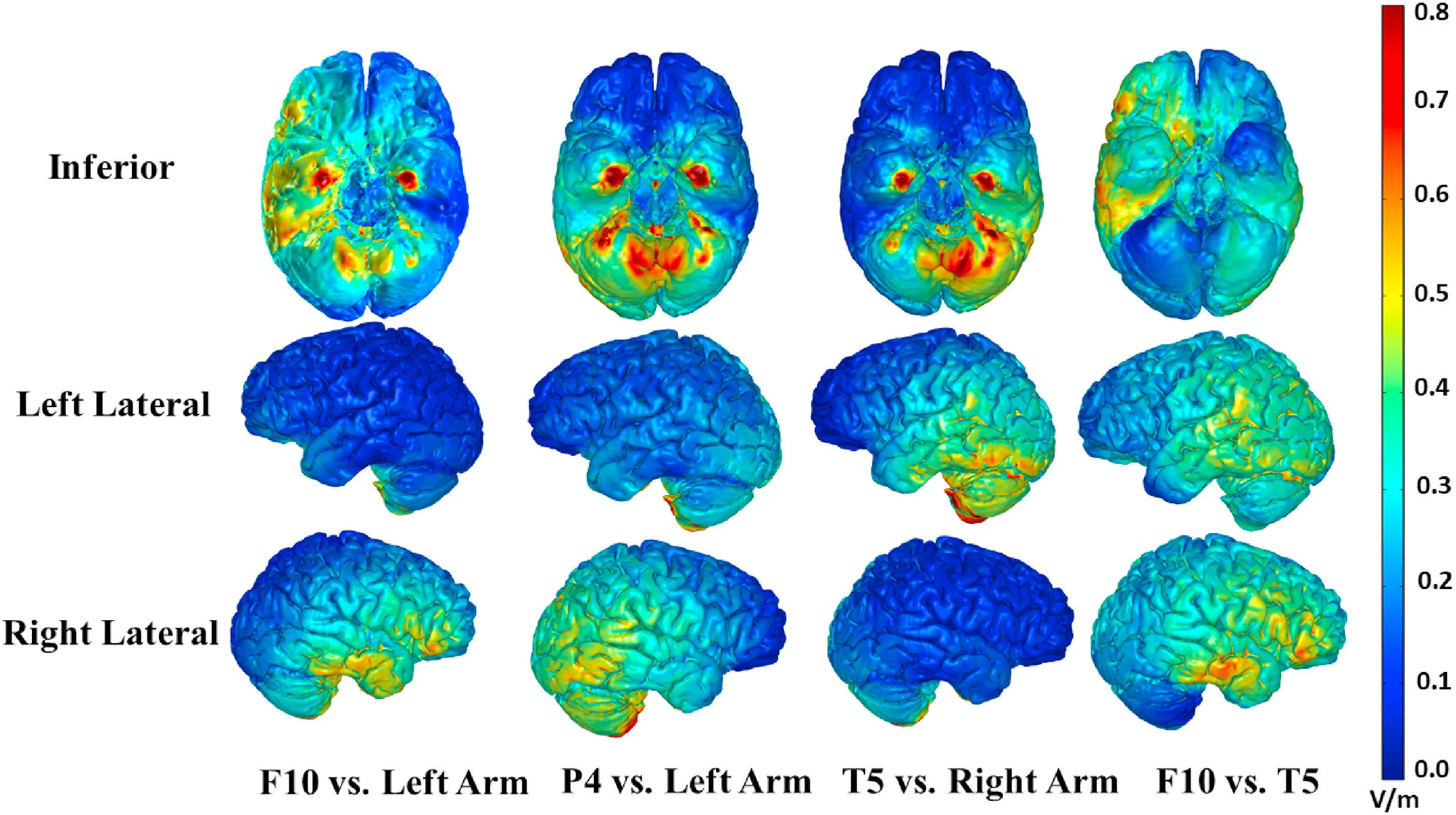
Abstract: Transcranial direct current stimulation (tDCS) influences performance in many cognitive domains. However, the question of which brain networks are involved in these effects is rarely examined. In prior experiments we identified tDCS protocols that produce a large improvement in category learning. Here we examined which brain regions were involved by modelling and comparing the behavioral effects of different electrode placements. In Experiment 1, we placed electrodes at two cephalic sites found the be most effective in our prior studies (F10 and T5/P7), expecting an increased combined effect. However, no effect was found, suggesting that stimulation of additional far field regions using extracephalic electrodes in our prior studies may have been necessary for producing these effects. In Experiment 2, we used finite element modeling (FEM) to compare the E-fields produced by these montages. One region with large differences and that is accessible to tDCS was the cerebellum. We then tested the involvement of the cerebellum by placing electrodes below the inion vs. the left arm in thirty-six participants who received anodal, cathodal, or sham stimulation during training. Neither anodal nor cathodal cerebellar tDCS led to significant changes when compared with sham. These results suggest that neither far-field stimulation of the cerebellum nor nearby cranial nerves played a large causal role in our previous tDCS studies. To our knowledge, this one of the first studies to systematically compare the behavioral and energetic effects produced by different montages to identify the specific brain regions involved in the behavioral responses to tDCS.
FEM of electrode placements used in previous studies (left 3 columns), and experiment 1 of the current study (rightmost columns). The first row shows an inferior view for each montage. The second row shows a left lateral view. The third row shows a right lateral view. Note that behavioral effects of tDCS were observed in the first three montages, but not in the fourth.
New publication in Brain Stimulation
Adaptive current-flow models of ECT: Explaining individual static impedance, dynamic impedance, and brain current density
Gozde Unal , Jaiti K. Swami, Carliza Canela, Samantha L. Cohen, Niranjan Khadka, Mohamad FallahRad, Baron Short, Miklos Argyelan, Harold A. Sackeim & Marom Bikson
Brain Stimulation | (2021) 14(5):1154-1168 | https://doi.org/10.1016/j.brs.2021.07.012
Download PDF
Abstract: Background: Improvements in electroconvulsive therapy (ECT) outcomes have followed refinement in device electrical output and electrode montage. The physical properties of the ECT stimulus, together with those of the patient's head, determine the impedances measured by the device and govern current delivery to the brain and ECT outcomes. Objective: However, the precise relations among physical properties of the stimulus, patient head anatomy, and patient-specific impedance to the passage of current are long-standing questions in ECT research and practice. To this end, we develop a computational framework based on diverse clinical data sets. Methods: We developed anatomical MRI-derived models of transcranial electrical stimulation (tES) that included changes in tissue conductivity due to local electrical current flow. These “adaptive” models simulate ECT both during therapeutic stimulation using high current (~1 A) and when dynamic impedance is measured, as well as prior to stimulation when low current (~1 mA) is used to measure static impedance. We modeled two scalp layers: a superficial scalp layer with adaptive conductivity that increases with electric field up to a subject-specific maximum (sSS), and a deep scalp layer with a subject-specific fixed conductivity (sDS). Results: We demonstrated that variation in these scalp parameters may explain clinical data on subjectspecific static impedance and dynamic impedance, their imperfect correlation across subjects, their relationships to seizure threshold, and the role of head anatomy. Adaptive tES models demonstrated that current flow changes local tissue conductivity which in turn shapes current delivery to the brain in a manner not accounted for in fixed tissue conductivity models. Conclusions: Our predictions that variation in individual skin properties, rather than other aspects of anatomy, largely govern the relationship between static impedance, dynamic impedance, and ECT current delivery to the brain, themselves depend on assumptions about tissue properties. Broadly, our novel modeling pipeline opens the door to explore how adaptive-scalp conductivity may impact transcutaneous electrical stimulation (tES).
July 22, 2021 . (8-9 AM ET)n Prof. Marom Bikson speaks (online) to Justus-Liebig-University Gieße on “Technology and fundamentals of tACS”
Slides: PDF