NYC Neuromodulation Online 2020 Conference will be a virtual, self-organized and self-managed meeting spanning 3 days. The conference will run continuously from April 20th at 9 AM (ET) to April 22nd 9 PM (ET). Any member of the scientific community can develop and submit a session and registration is free of charge and open to everyone.
All meetings must be conducted via Zoom video-conferencing software. After an organizer submits a session, it goes into the review phase by our Conference Scientific Committee. When the session is approved it is posted on our website and participants can start reserving their seats.
NYC Neuromodulation started as a grass-roots non-profit meeting first held in 2015 at the City College of New York in New York City. In 2017, NYC Neuromodulation was run again at The City College of New York. In 2018, NYC Neuromodulation was run jointly with the North American Neuromodulation Society (NANS) at the Sheraton, New York City, In 2019, NYC Neuromodulation was run jointly with the Neuromodulation: The Science conference in Napa, California. The NYC Neuromodulation Online 2020 conference continues in grass-roots spirit, opening up the conference attendance and organization to the neuromodulation community.
New publication:
Front. Hum. Neurosci., 18 March 2020 | https://doi.org/10.3389/fnhum.2020.00077
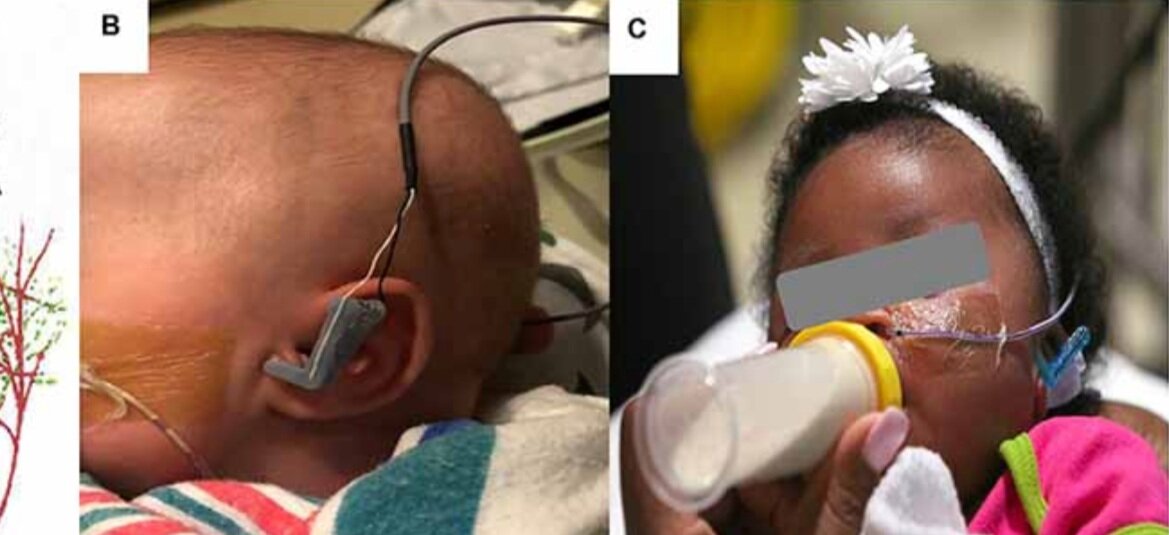
Transcutaneous Auricular Vagus Nerve Stimulation-Paired Rehabilitation for Oromotor Feeding Problems in Newborns: An Open-Label Pilot Study
Bashar W. Badran, Dorothea D. Jenkins, Daniel Cook, Sean Thompson, Morgan Dancy, William H. DeVries, Georgia Mappin, Philipp Summers, Marom Bikson and Mark S. George
Abstract: Neonates born premature or who suffer brain injury at birth often have oral feeding dysfunction and do not meet oral intake requirements needed for discharge. Low oral intake volumes result in extended stays in the hospital (>2 months) and can lead to surgical implant and explant of a gastrostomy tube (G-tube). Prior work suggests pairing vagus nerve stimulation (VNS) with motor activity accelerates functional improvements after stroke, and transcutaneous auricular VNS (taVNS) has emerged as promising noninvasive form of VNS. Pairing taVNS with bottle-feeding rehabilitation may improve oromotor coordination and lead to improved oral intake volumes, ultimately avoiding the need for G-tube placement. We investigated whether taVNS paired with oromotor rehabilitation is tolerable and safe and facilitates motor learning in infants who have failed oral feeding. We enrolled 14 infants [11 premature and 3 hypoxic–ischemic encephalopathy (HIE)] who were slated for G-tube placement in a prospective, open-label study of taVNS-paired rehabilitation to increase feeding volumes. Once-daily taVNS was delivered to the left tragus during bottle feeding for 2 weeks, with optional extension. The primary outcome was attainment of oral feeding volumes and weight gain adequate for discharge without G-tube while also monitoring discomfort and heart rate (HR) as safety outcomes. We observed no adverse events related to stimulation, and stimulation-induced HR reductions were transient and safe and likely confirmed vagal engagement. Eight of 14 participants (57%) achieved adequate feeding volumes for discharge without G-tube (mean treatment length: 16 ± 6 days). We observed significant increases in feeding volume trajectories in responders compared with pre-stimulation (p < 0.05). taVNS-paired feeding rehabilitation appears safe and may improve oral feeding in infants with oromotor dyscoordination, increasing the rate of discharge without G-tube, warranting larger controlled trials.
Held online (following Covid-19 concerns)
Dr. Marom Bikson speaks on “Modeling of Brain & Cranial Nerve Activation”. Download the sides here
Conference and viewing information here
Conference overview: Non-invasive vagus nerve stimulation (VNS) may be administered via a novel, emerging neuromodulatory technique known as transcutaneous auricular vagus nerve stimulation (taVNS). Unlike cervically-implanted VNS, taVNS is an inexpensive and non-surgical alternative to modulate the vagus nerve. taVNS delivers electrical stimulation to the auricular branch of the vagus nerve (ABVN), an easily accessible target that innervates the human ear and is appealing as it allows for rapid translation of basic VNS research. Interest in taVNS has grown over the past decade with many promising neuropsychiatric disease applications such as stroke and depression, as well as enhancing neuroplasticity and reducing inflammation. This safe, inexpensive, and portable neurostimulation modality has major implications in the future treatment of central and peripheral disease. This 2-day conference, organized by recognized taVNS expert Dr. Bashar Badran, is designed to provide lectures by international taVNS experts and hands-on opportunities to learn and practice the fundamentals of noninvasive vagus nerve stimulation.
New publication and technology:
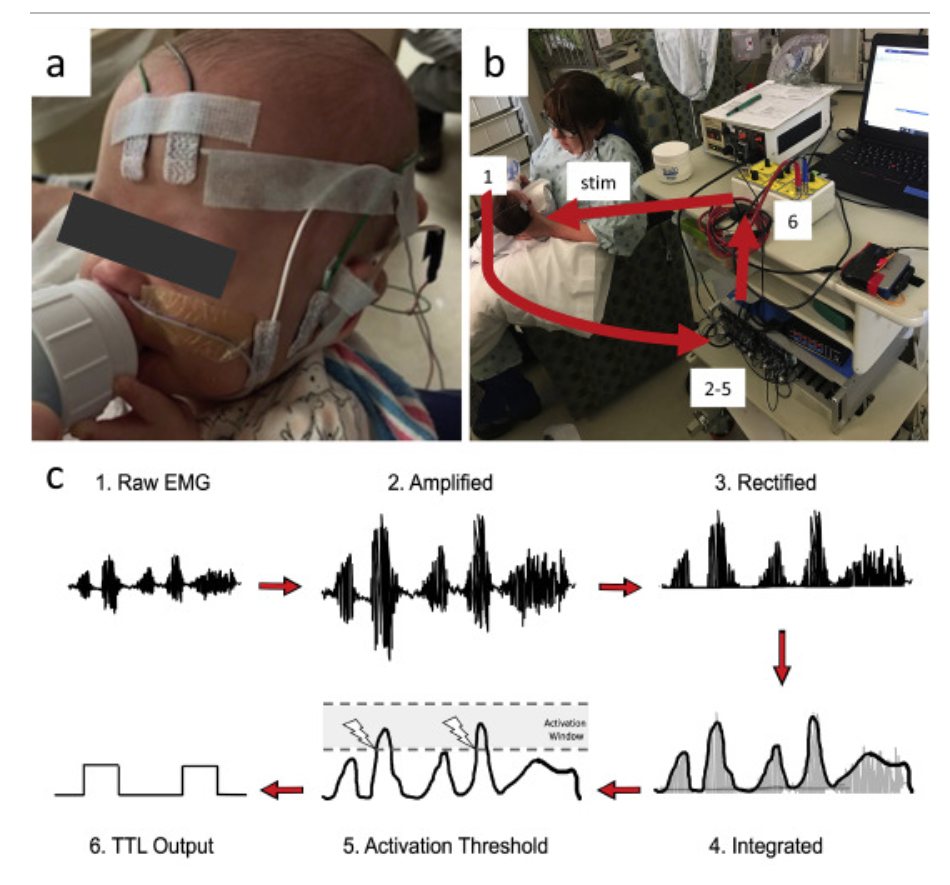
Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation
Daniel N. Cook , Sean Thompson , Sasha Stomberg-Firestein , Marom Bikson, Mark S. George , Dorothea D. Jenkins , Bashar W. Badran
Abstract: Background: Studies have found that pairing vagus nerve stimulation (VNS) with motor activity accelerates cortical reorganization. This synchronous pairing may enhance motor recovery. Objective: To develop and validate a motor-activated auricular vagus nerve stimulation (MAAVNS) system as a potential neurorehabilitation tool. Methods: We created MAAVNS and validated its function as part of an ongoing clinical trial investigating whether taVNS-paired rehabilitation enhances oromotor learning. We compared 3 different MAAVNS EMG electrode configurations in 3 neonates. The active lead was placed over the buccinator muscle. Reference lead placements were orbital, temporal or frontal. Results: The frontal reference lead produced the highest sensitivity (0.87 ± 0.07 (n ¼ 8)) and specificity (0.64 ± 0.13 (n ¼ 8)). Oral sucking reliably triggers MAAVNS stimulation with high confidence. Conclusion: EMG electrodes placed on target orofacial muscles can effectively trigger taVNS stimuli in infants in a closed loop fashion.
Prof. Marom Bikson hosts the first 2020 Jake Zabara Lectureship in NeuroCybernetic Modulation
Wednesday, February 26, 2020, 3:00-4:00 PM, Reception Follows, Location: ASRC Auditorium
Invited 2020 Jake Zabara Lectureship in NeuroCybernetic Modulation speaker: Brian H. Kopell, MD
" The nervous system is not a light switch-VNS, Neuromodulation and Neuroplasticity”
Dr. Brian Kopell is a Professor of Neurosurgery, Neurology, Psychiatry, and Neuroscience and the Director of the Center for Neuromodulation at the Mount Sinai Health System. Dr. Kopell has pioneered the use of neuromodulation technologies including Vagus Nerve Stimulation (VNS) and Deep Brain Stimulation (DBS) for the treatment of neurological and psychiatric disorders. Dr. Kopell will give the first annual Jake Zabara Lectureship in NeuroCybernetic Modulation on the history, current clinical impact, and future development of VNS.
With opening remarks by Dr. Jake Zabara. Dr. Zabara is the inventor of Vagus Nerve Stimulation (VNS) therapy, commercialized for the treatment of Epilepsy and Depression by LivaNova (formally Cyberonics).
Update- Images from Event:
New lab publication: Impact of brain atrophy on tDCS and HD-tDCS current flow: a modeling study in three variants of primary progressive aphasia.
Neurological Sciences https://doi.org/10.1007/s10072-019-04229-z
Gozde Unal, Bronte Ficek, Kimberly Webster, Syed Shahabuddin, Dennis Truong, Benjamin Hampstead, Marom Bikson, Kyrana Tsapkini
Download PDF
Abstract: Background During transcranial direct current stimulation (tDCS), the amount and distribution of current that reaches the brain depends on individual anatomy. Many progressive neurodegenerative diseases are associated with cortical atrophy, but the importance of individual brain atrophy during tDCS in patients with progressive atrophy, including primary progressive aphasia (PPA), remains unclear. Objective In the present study, we addressed the question whether brain anatomy in patients with distinct cortical atrophy patterns would impact brain current intensity and distribution during tDCS over the left IFG. Method We developed state-of-the-art, gyri-precise models of three subjects, each representing a variant of primary progressive aphasia: non-fluent variant PPA (nfvPPA), semantic variant PPA (svPPA), and logopenic variant PPA (lvPPA). We considered two exemplary montages over the left inferior frontal gyrus (IFG): a conventional pad montage (anode over F7, cathode over the right cheek) and a 4 × 1 high-definition tDCS montage. We further considered whether local anatomical features, specifically distance of the cortex to skull, can directly predict local electric field intensity. Results We found that the differences in brain current flow across the three PPA variants fall within the distribution of anatomically typical adults. While clustering of electric fields was often around individual gyri or sulci, the minimal distance from the gyri/sulci to skull was not correlated with electric field intensity. Conclusion Limited to the conditions and assumptions considered here, this argues against a specific need to adjust the tDCS montage for these patients any more than might be considered useful in anatomically typical adults. Therefore, local atrophy does not, in isolation, reliably predict local electric field. Rather, our results are consistent with holistic head anatomy influencing brain current flow, with tDCS producing diffuse and individualized brain current flow patterns and HD-tDCS producing targeted brain current flow across individuals.
Niranjan Khadka, Irene E. Harmsen, Andres M. Lozano, Marom Bikson. Bio-Heat Model of Kilohertz-Frequency Deep Brain Stimulation Increases Brain Tissue Temperature. Neuromodulation 2020. DOI: 10.1111/ner.13120
Download PDF published in Neuromodulation — DOI
Abstract
Objectives
Early clinical trials suggest that deep brain stimulation at kilohertz frequencies (10 kHz‐DBS) may be effective in improving motor symptoms in patients with movement disorders. The 10 kHz‐DBS can deliver significantly more power in tissue compared to conventional frequency DBS, reflecting increased pulse compression (duty cycle). We hypothesize that 10 kHz‐DBS modulates neuronal function through moderate local tissue heating, analogous to kilohertz spinal cord stimulation (10 kHz‐SCS). To establish the role of tissue heating in 10 kHz‐DBS (30 μs, 10 kHz, at intensities of 3‐7 mApeak), a decisive first step is to characterize the range of temperature changes during clinical kHz‐DBS protocols.
Materials and Methods
We developed a high‐resolution magnetic resonance imaging‐derived DBS model incorporating joule‐heat coupled bio‐heat multi‐physics to establish the role of tissue heating. Volume of tissue activated (VTA) under assumptions of activating function (for 130 Hz) or heating (for 10 kHz) based neuromodulation are contrasted.
Results
DBS waveform power (waveform RMS) determined joule heating at the deep brain tissues. Peak heating was supralinearly dependent on stimulation RMS. The 10 kHz‐DBS stimulation with 2.3 to 5.4 mARMS (corresponding to 3 to 7 mApeak) produced 0.10 to 1.38°C heating at the subthalamic nucleus (STN) target under standard tissue parameters. Maximum temperature increases were predicted inside the electrode encapsulation layer (enCAP) with 2.3 to 5.4 mARMS producing 0.13 to 1.87°C under standard tissue parameters. Tissue parameter analysis predicted STN heating was especially sensitive (ranging from 0.44 to 1.35°C at 3.8 mARMS) to decreasing enCAP electrical conductivity and decreasing STN thermal conductivity.
Conclusions
Subject to validation with in vivo measurements, neuromodulation through a heating mechanism of action by 10 kHz‐DBS can indicate novel therapeutic pathways and strategies for dose optimization.
Dr. Marom Bikson co-direct (with Dr. Scott Lempka) the North American Neuromodulation Society (NANS) pre-conference workshop, “Engineering principles of spinal cord stimulation and deep brain stimulation for clinicians,” from 8:00am to 5:00pm on Thursday January 23, 2020 at Caesars Palace in Las Vegas, NV.
The exciting workshop will present engineering principles relevant to Spinal Cord Stimulation (SCS) and Deep Brain Stimulation (DBS). The target audience is clinicians that currently utilize or are interested in incorporating SCS and/or DBS technologies into their clinical practice. The field of neuromodulation is rapidly evolving and several technologies are now clinically available. These systems have diverse lead and stimulator designs. It is not always clear how different designs or waveform parameters affect the neural response and corresponding efficacy of the stimulation. Therefore, the goal of this course is to provide clinicians with an overview of the engineering principles and biophysics relevant to SCS and DBS. This course will also present the current understanding of the physiological effects and mechanisms of action of standard and emerging forms of SCS and DBS. Furthermore, this course will describe safety and regulatory issues that are critical to electrode design and stimulation parameters. At the end of the course, attendees will have a better understanding of the physiological and technical factors that determine the neural response to SCS and DBS. The ultimate goal of this course is to provide attendees with knowledge that will aid in their clinical implementation of SCS and DBS
Dr. Bikson provides two lectures in the course:
08:10 – 08:50 am: Neurostimulation fundamentals – waveform basics, membrane polarization, action potential threshold, region of activation. Download the lecture slides
02:00 – 02:30 pm: Sub-threshold mechanisms and heating in Spinal Cord Stimulation (SCS) and Deep Brain Stimulation (DBS) Download the lecture slides
Title: Modern Electroconvulsive Therapy: Vastly Improved Yet Greatly Underused
Speaker: Dr. Harold Sackeim, Professor of Psychiatry and Radiology at Columbia University and Chief of Biological Psychiatry at the New York State Psychiatric Institute
When: Thursday, February 13, 2020, 10 am to 12 noon
Where: CCNY Center for Discovery and Innovation, 3rd floor seminar room (CDI 3.352)
Contact: Marom Bikson (bikson@ccny.cuny.edu, 212-650-6791) for access to CDI building
Biography:
Dr. Harold A. Sackeim is Professor of Clinical Psychology in Psychiatry and Radiology, College of Physicians and Surgeons, Columbia University. He served as Chief of the Department of Biological Psychiatry at the New York State Psychiatric Institute for 25 years. He is also the Founding Editor of the journal, Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. He received his first B.A. from Columbia College, Columbia University (1972), another B.A. and a M.A. from Magdalen College, Oxford University (1974) and his Ph.D. from the University of Pennsylvania (1977), where he also completed his clinical training in the Department of Psychiatry. He joined the faculty of Columbia University in 1977, where he remains today.
His research has concentrated on the neurobiology and treatment of mood disorders. He has made numerous contributions to the understanding of pathophysiology of major depression and mania through use of brain imaging techniques and by examining the role of lateralization of brain function in normal emotion, neurological disorders, and psychiatric illness. For 30 years, he led the clinical research on electroconvulsive therapy (ECT) at Columbia University and the New York State Psychiatric Institute. This work has identified fundamental factors in this treatment that are responsible for its efficacy and side effects, and has radically altered understanding of both therapeutics and mechanisms of action. This research program has provided compelling evidence regarding the localization of the brain circuits involved in antidepressant effects, and has revamped understanding of the underpinnings of ECT’s effects on mood, behavior, and cognition. Dr. Sackeim is widely credited with transforming the use of this treatment worldwide.
Dr. Sackeim has directed programs at the New York State Psychiatric Institute and New York Presbyterian Hospital in the pharmacological treatment of late-life depression, and in the use of Transcranial Magnetic Stimulation (TMS), Vagus Nerve Stimulation (VNS), Deep Brain Stimulation (DBS) and other forms of focal brain stimulation. Dr. Sackeim is the inventor of Magnetic Seizure Therapy (MST), now undergoing clinical trials and has recently developed FEAST (Focal Electrically-Administered Seizure Therapy), now also in clinical trials. Dr. Sackeim introduced functional brain imaging to the medical center at Columbia in 1980, and directed a large group using Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) to study pathophysiology and treatment effects in mood disorders, anxiety disorders, Lyme disease, substance abuse, Alzheimer’s disease, and normal aging. Other work directed by Dr. Sackeim involved preclinical, primate research on the functional significance of structural brain changes (neurogenesis) induced by different forms of brain stimulation.
Dr. Sackeim is a member of the editorial board of several journals, and has received many national and international awards for his research contributions. These include three Distinguished Investigator Awards from the National Association for Research in Schizophrenia and Depression (NARSAD), a MERIT Award from the National Institute of Mental Health (NIMH), the Joel Elkes International Award from the American College of Neuropsychopharmacology (ACNP), election as Honorary Fellow of the American Psychiatric Association, and the Award for Research Excellence from the New York State Office of Mental Hygiene, Edward Smith Lectureship, National Institute of Psychobiology, Israel, the lifetime achievement award form the EEG and CNS Society, and the NARSAD Maddox Falcone Prize for lifetime achievement in research on affective disorders. He is past President of the Society of Biological Psychiatry and the Association for Research in Nervous and Mental Disease. He has authored more than 450 publications.
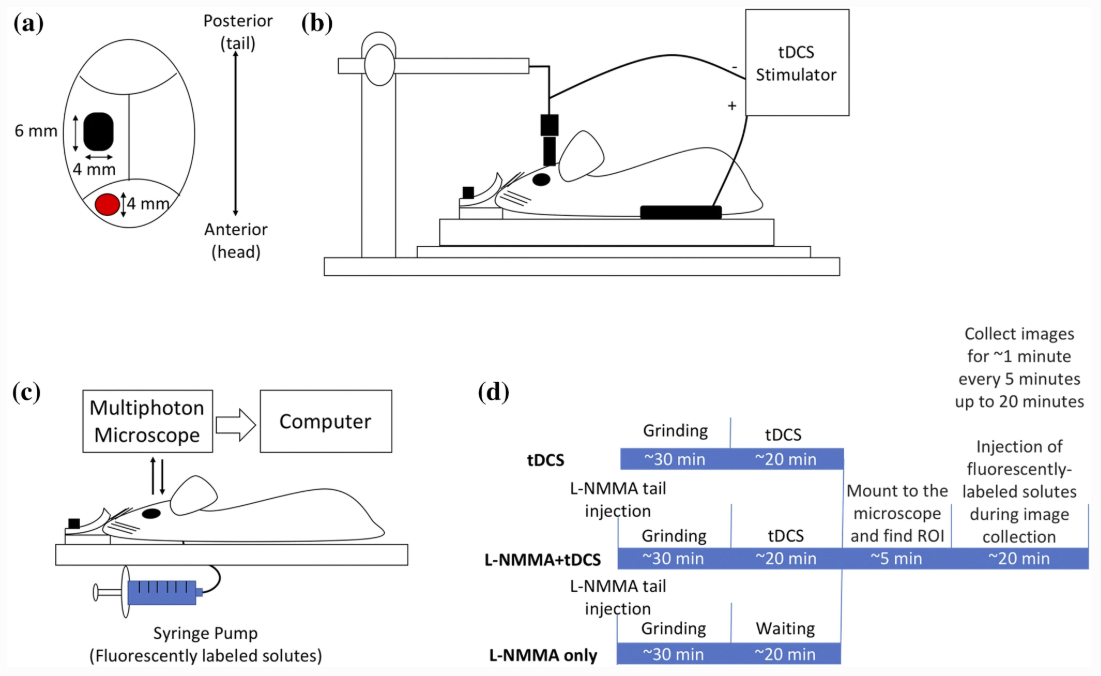
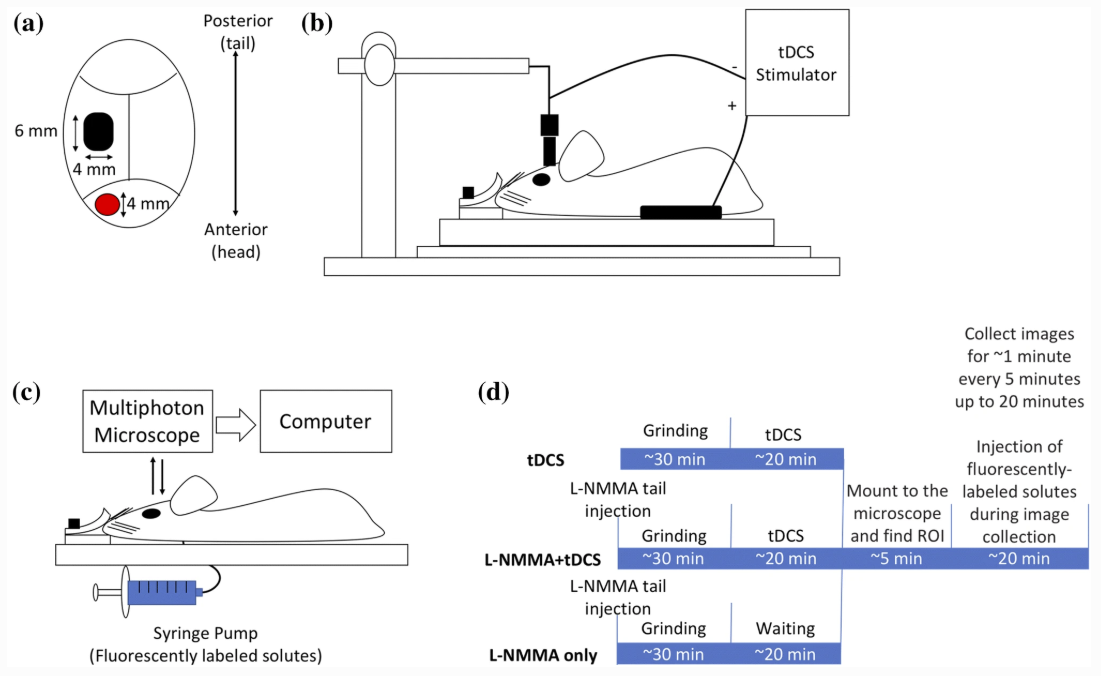
Shin DW, Fan J, Luu E, Khalid W, Xia Y, Khadka N, Bikson M, Fu BM. In Vivo Modulation of the Blood–Brain Barrier Permeability by Transcranial Direct Current Stimulation (tDCS). Annals of Biomedical Engineering 2020. https://doi.org/10.1007/s10439-020-02447-7
Download PDF published in Annals of Biomedical Engineering — DOI
Abstract
tDCS has been used to treat various brain disorders and its mechanism of action (MoA) was found to be neuronal polarization. Since the blood–brain barrier (BBB) tightly regulates the neuronal micro-environment, we hypothesized that another MoA of tDCS is direct vascular activation by modulating the BBB structures to increase its permeability (P). To test this hypothesis, we used high resolution multi-photon microscopy to determine P of the cerebral micro vessels in rat brain. We found that 20 min 0.1– 1 mA tDCS transiently increases P to a small solute, sodium fluorescein (MW 376) and to a large solute, Dextran-70k, with a much higher increase in P to the large solute. By pretreating the vessel with a nitric oxide synthase inhibitor, we revealed that the tDCS-induced increase in P is NO dependent. A transport model for the BBB was further employed to predict the structural changes by the tDCS. Comparing model predictions with the measured data suggests that tDCS increases P by temporarily disrupting the structural components forming the paracellular pathway of the BBB. That the transient and reversible increase in the BBB permeability also suggests new applications of tDCS such as a non-invasive approach for brain drug delivery through the BBB.
Borges H, Dufau A, Paneri B, Woods AJ, Knotkova H, Bikson M. Updated Technique for Reliable, Easy, and Tolerated Transcranial Electrical Stimulation Including Transcranial Direct Current Stimulation. Journal of Visualized Experiments 2020. https://doi.org/10.3791/59204.
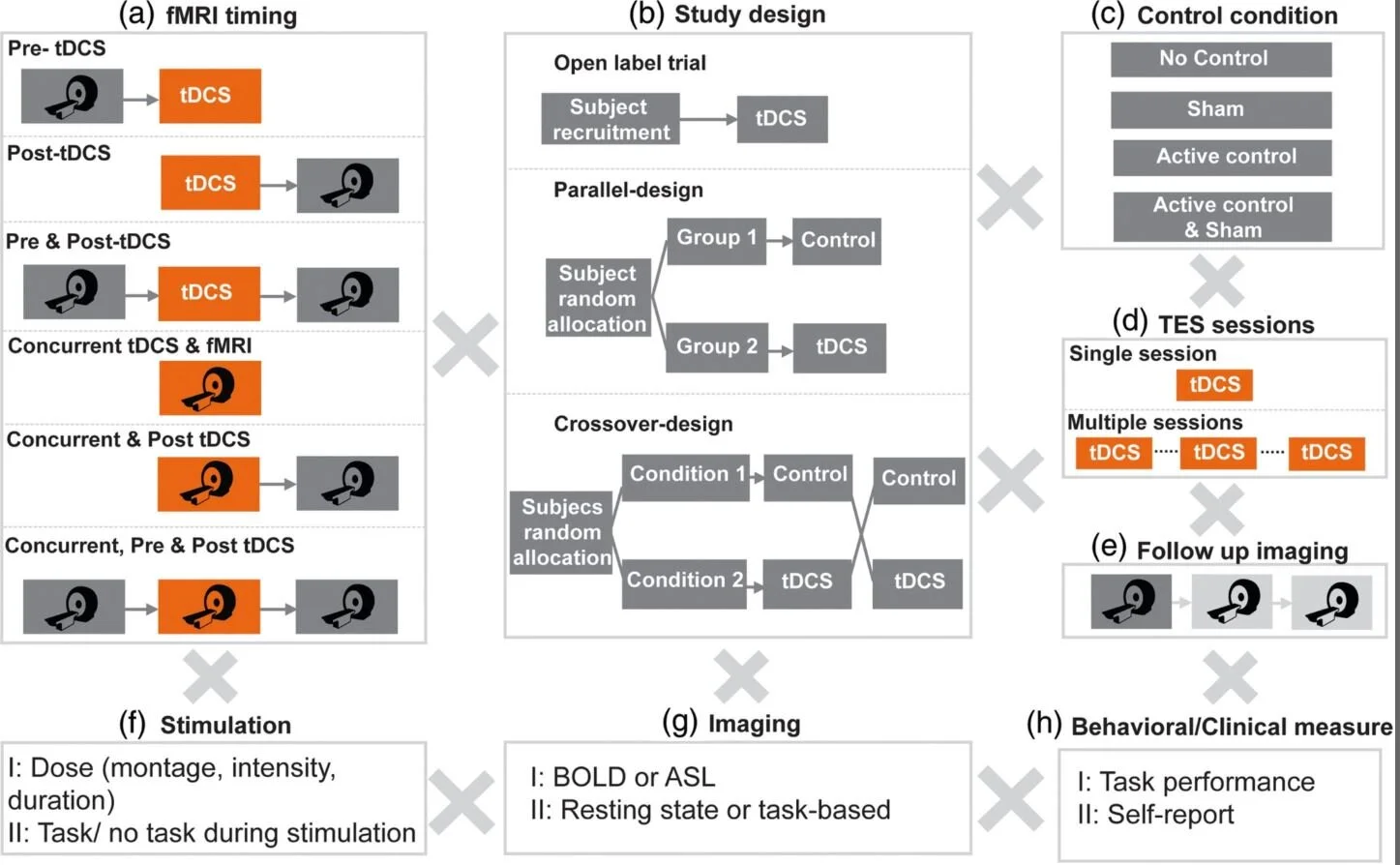
Esmaeilpour Z, Shereen AD, Ghobadi‐Azbari P, Datta A, Woods AJ, Ironside M, O’Shea J, Kirk U, Bikson M, Ekhtiari H. Methodology for tDCS integration with fMRI. Human Brain Mapping. 2019 Dec 24; Available from: http://dx.doi.org/10.1002/hbm.24908
Download PDF published in Human Brain Mapping — DOI
Abstract
Understanding and reducing variability of response to transcranial direct current stimulation (tDCS) requires measuring what factors predetermine sensitivity to tDCS and tracking individual response to tDCS. Human trials, animal models, and computational models suggest structural traits and functional states of neural systems are the major sources of this variance. There are 118 published tDCS studies (up to October 1, 2018) that used fMRI as a proxy measure of neural activation to answer mechanistic, predictive, and localization questions about how brain activity is modulated by tDCS. FMRI can potentially contribute as: a measure of cognitive state‐level variance in baseline brain activation before tDCS; inform the design of stimulation montages that aim to target functional networks during specific tasks; and act as an outcome measure of functional response to tDCS. In this systematic review, we explore methodological parameter space of tDCS integration with fMRI spanning: (a) fMRI timing relative to tDCS (pre, post, concurrent); (b) study design (parallel, crossover); (c) control condition (sham, active control); (d) number of tDCS sessions; (e) number of follow up scans; (f) stimulation dose and combination with task; (g) functional imaging sequence (BOLD, ASL, resting); and (h) additional behavioral (cognitive, clinical) or quantitative (neurophysiological, biomarker) measurements. Existing tDCS‐fMRI literature shows little replication across these permutations; few studies used comparable study designs. Here, we use a representative sample study with both task and resting state fMRI before and after tDCS in a crossover design to discuss methodological confounds. We further outline how computational models of current flow should be combined with imaging data to understand sources of variability. Through the representative sample study, we demonstrate how modeling and imaging methodology can be integrated for individualized analysis. Finally, we discuss the importance of conducting tDCS‐fMRI with stimulation equipment certified as safe to use inside the MR scanner, and of correcting for image artifacts caused by tDCS. tDCS‐fMRI can address important questions on the functional mechanisms of tDCS action (e.g., target engagement) and has the potential to support enhancement of behavioral interventions, provided studies are designed rationally.
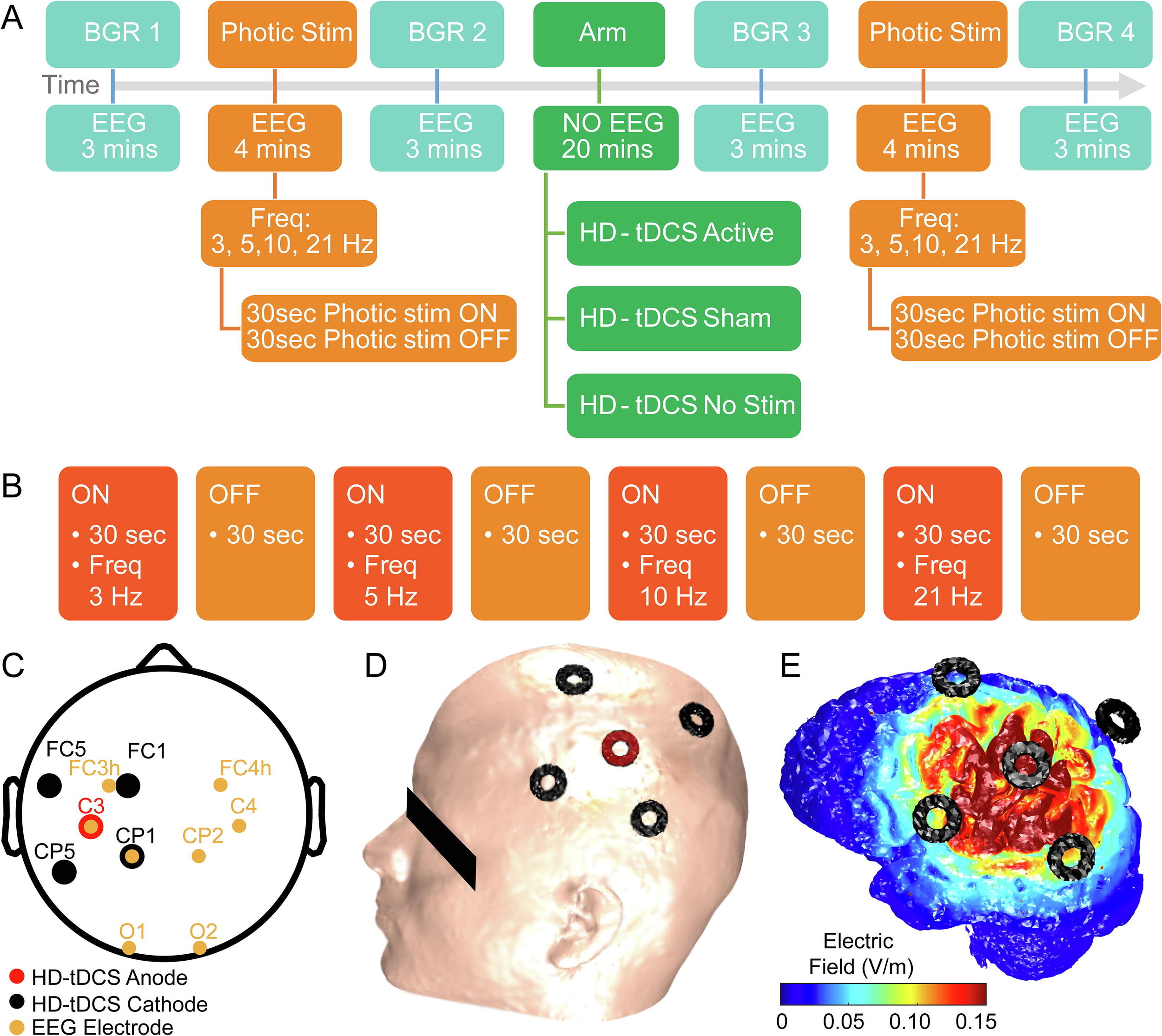
Vladimir V. Lazarev, Nigel Gebodh, Tiago Tamborino, Bikson, M, & Egas Caparelli-Daquer. (2020). Experimental-design Specific Changes in Spontaneous EEG and During Intermittent Photic Stimulation by High Definition Transcranial Direct Current Stimulation. Neuroscience, 426, 50–58. https://doi.org/10.1016/j.neuroscience.2019.11.016
Download PDF published in Journal Neuroscience Methods— DOI
Abstract
Electroencephalography (EEG) as a biomarker of neuromodulation by High Definition transcranial Direct Current Stimulation (HD-tDCS) offers promise as both techniques are deployable and can be integrated into a single head-gear. The present research addresses experimental design for separating focal EEG effect of HD-tDCS in the ‘4-cathode × 1-anode’ (4 × 1) montage over the left motor area (C3). We assessed change in offline EEG at the homologous central (C3, C4), and occipital (O1, O2) locations. Interhemispheric asymmetry was accessed for background EEG at standard frequency bands; and for the intermittent photic stimulation (IPS). EEG was compared post- vs pre-intervention in three HD-tDCS arms: Active (2 mA), Sham (ramp up/down at the start and end), and No-Stimulation (device was not powered), each intervention lasting 20 min. The asymmetric background EEG changes were only in the central areas with right-side amplitude spectra prevalence, most pronounced in the no-stimulation arm, where they depended on comparison time-points and were consistent with markers of transition between drowsiness and vigilance – bilateral decrease in the delta and asymmetric central increase in the alpha and beta1 bands. For the active arm, similar but less pronounced changes occurred in the alpha band. In contrast, responses to IPS developed similar asymmetric amplitude increase at four harmonics of the IPS of 3 Hz only in the active arm, against a background of a brain-wide symmetric increase in both active and sham arms. Our protocols and analyses suggest methodological caveats for how EEG of tDCS studies could be conducted to isolate putative brain polarization outcomes.
Niranjan Khadka, Marom Bikson. Response to the Letter to the Editor by Caraway et al. on “Tissue Temperature Increases by a 10 kHz Spinal Cord Stimulation System: Phantom and Bioheat Model”. Neuromodulation 2019. https://doi.org/10.1111/ner.13079. PDF
Download: PDF published in Neuromodulation - DOI
To the Editor:
We would like to respond to the Letter to the Editor by Dr. Caraway, Dr. Bradley, and Dr. Lee regarding our recent paper “Tissue Temperature Increases by a 10 kHz Spinal Cord Stimulation System: Phantom and Bioheat Model” 1. Caraway et al. correctly described the explicit aim of our paper “to explore the role of joule heating as a mechanism of action for HF10 therapy.” Caraway et al. also confirmed that we “conclude that the measured temperature changed in a predictable manner due to the physics of electrical heating in a volume conductor.” We then predicted temperature increases at the spinal cord and near the lead using a bioheat FEM model.
We noted in our paper that “the bioheat models of kHz SCS remain to be validated” and Caraway et al. emphasized that we did not include in vivo (preclinical or clinical) data. In this paper, and our prior publication on the general topic “Temperature Increases by kilohertz frequency Spinal Cord Stimulation” 2, we explicitly considered how computational models' assumption may increase or decrease predicted temperature rises. If ongoing validation confirms heating of ~1°C, there are key outstanding questions on if and how pain processing pathways are affected. In the spirit of “meritorious scientific discussion,” we may differ with Caraway et al. on the potential impact of moderate heating as a mechanism of action and how this supports findings from clinical trials. But, we agree it is a misinterpretation of our work to suggest a prediction of ~1°C heating is evidence disproving preclinical or clinical data on the safety of HF10.
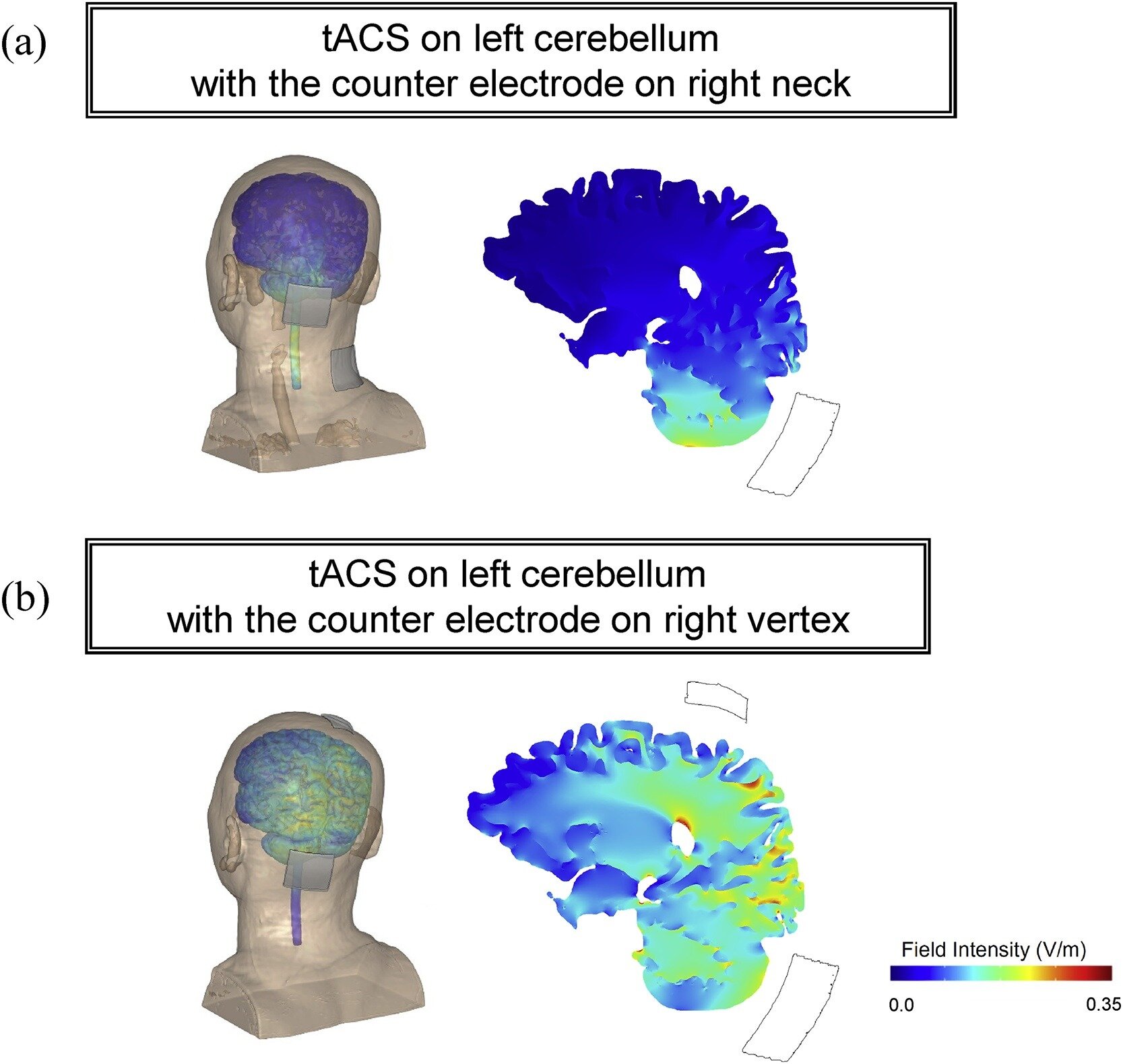
Koganemaru S, Mikami Y, Matsuhashi M, Truong DQ, Bikson M, Kansaku K, Mima T. Cerebellar transcranial alternating current stimulation modulates human gait rhythm. Neuroscience Research. 2019 Dec; Available from: http://dx.doi.org/10.1016/j.neures.2019.12.003
Download PDF published in Neuroscience Research — DOI
Abstract
Although specific brain regions are important for regularly patterned limb movements, the rhythm generation system that governs bipedal locomotion in humans is not thoroughly understood. We investigated whether rhythmic transcranial brain stimulation over the cerebellum could alter walking rhythm. Fourteen healthy subjects performed over-ground walking for 10 min during which they were given, in a random order, transcranial alternating current stimulation (tACS) over the left cerebellum at the approximated frequency of their gait cycle, tACS over the skin of the scalp, and during sham stimulation. Cerebellar tACS showed a significant entrainment of gait rhythm compared with the control conditions. When the direction of the tACS currents was symmetrically inverted, some subjects showed entrainment at an approximately 180° inverted phase, suggesting that gait modulation is dependent on current orientation. These findings indicate that tACS over cerebellum can modulate gait generation system in cerebellum and become an innovative approach for the recovery of locomotion in patients with gait disturbances caused by CNS disorders.
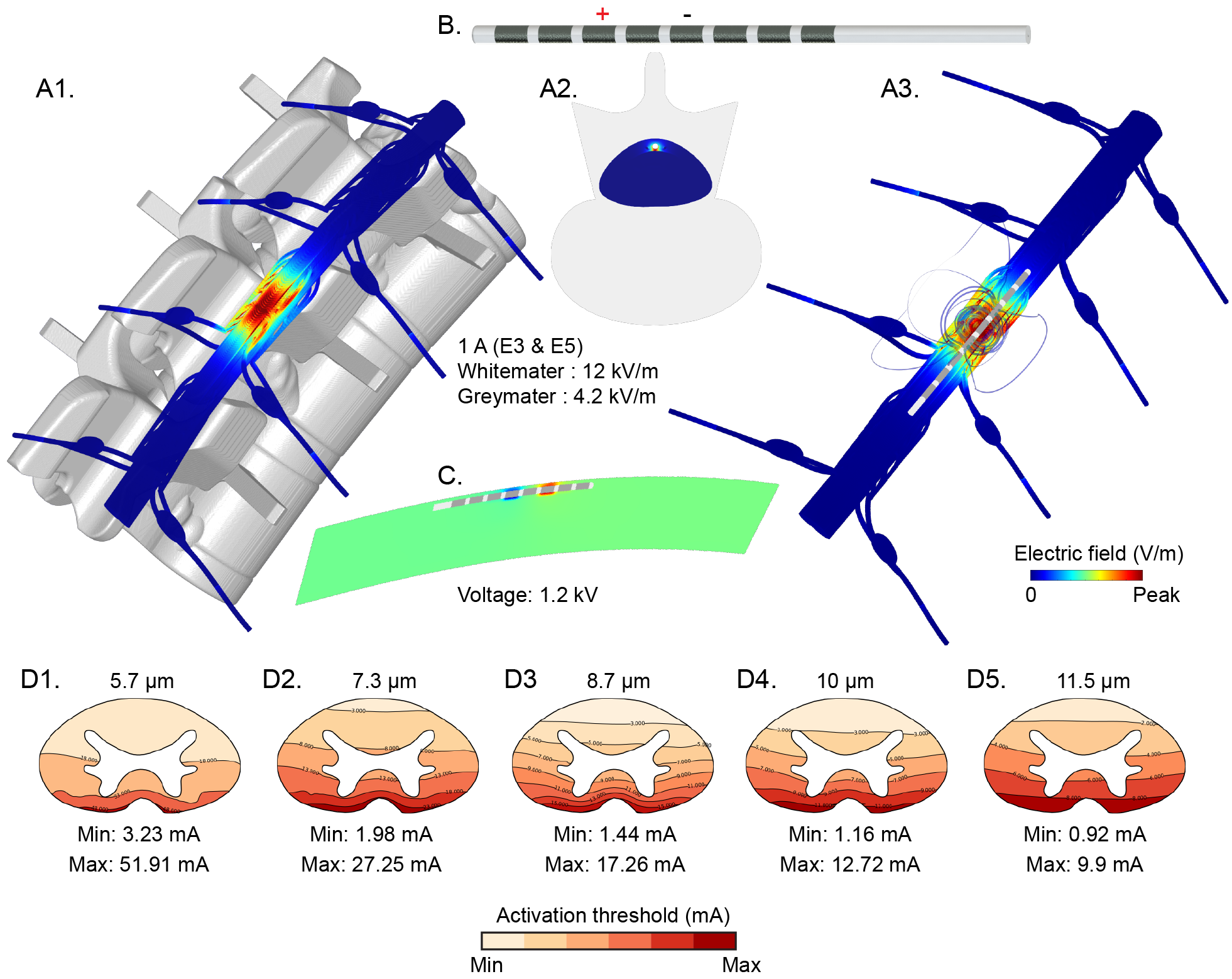
Niranjan Khadka, Xijie Liu, Hans Zander, Jaiti Swami, Evan Rogers, Scott F. Lempka, Marom Bikson. Realistic Anatomically Detailed Open-Source Spinal Cord Stimulation (RADO-SCS) Model. bioRxiv. 2019. https://doi.org/10.1101/857946
Download: PDF published in bioRxiv — DOI
Abstract
Objective: Computational current flow models of spinal cord stimulation (SCS) are widely used in device development, clinical trial design, and patient programming. Proprietary models of varied sophistication have been developed. An open-source model with state-of-the-art precision would serve as a standard for SCS simulation.
Approach: We developed a sophisticated SCS modeling platform, named Realistic Anatomically Detailed Open-Source Spinal Cord Stimulation (RADO-SCS) model. This platform consists of realistic and detailed spinal cord and ancillary tissues anatomy derived based on prior imaging and cadaveric studies. Represented tissues within the T9-T11 spine levels include vertebrae, intravertebral discs, epidural space, dura, CSF, white-matter, gray-matter, dorsal and ventral roots and rootlets, dorsal root ganglion, sympathetic chain, thoracic aorta, epidural space vasculature, white-matter vasculature, and thorax. As an exemplary, a bipolar SCS montage was simulated to illustrate the model workflow from the electric field calculated from a finite element model (FEM) to activation thresholds predicted for individual axons populating the spinal cord.
Main Results: Compared to prior models, RADO-SCS meets or exceeds detail for every tissue compartment. The resulting electric fields in white and gray-matter, and axon model activation thresholds are broadly consistent with prior stimulations.
Significance: The RADO-SCS can be used to simulate any SCS approach with both unprecedented resolution (precision) and transparency (reproducibility). Freely available online, the RADO-SCS will be updated continuously with version control.
Update watch talk video here
Prof. Marom Bikson speaks at the Cleveland FES Center NEURAL PROTHESIS LIVE WEBINAR on Dec 5, 2019 at 3:00 PM. Live Stream at: http://fescenter.org/news-events/webinar-series/
Download presentation slides
Title: Neuromodulation though BBB stimulation or Heating: New Mechanisms of DBS, SCS, and tDCS
Abstract: This seminar explores when direct heating of tissue or direct stimulation of endothelial cells of the blood-brain barrier (BBB) is the the mechanisms of action in neuromodulation. Specific approaches considered are Spinal Cord Stimulation (SCS), kHz-frequency SCS, Deep Brain Stimulation (DBS), kHz-frequency DBS, and transcranial Direct Current Stimulation.
Results are based on computational modeling (including bio-heat FEM), phantoms, and animal models (including in vivo imaging, brain slices, and in vitro endothelial monolayers). Moderate (~1 C) non-injurious heating of tissue results from higher-power waveforms with implanted electrodes (kHZ SCS, high-density SCS, kHZ DBS). Especially when sustained over long periods, moderate heating will modulate brain function. Immediate and lasting non-injurious changes in BBB permeability can occur during SCS, DBS, and tDCS. Even small changes in BBB function can have profound effects on brain function. These results are shown to derive from well established physical principles such as joule heat and electroosmosis.
In some cases heating or BBB mechanisms operate parallel to traditional nervous tissue stimulation, and in others applications they may underpin responsiveness and so govern secondary neuronal changes.
DownloadL Flier
Morya E, Monte-Silva K, Bikson M, Esmaeilpour Z, Biazoli CE, Fonseca A, Bocci T, Farzan F, Chatterjee R, Hausdorff JM, da Silva Machado DG, Russowsky Brunoni A, Mezger E, Aparecida Mascaleski L, Pegado R, Sato JR, Caetano MS, Nunes Sá K, Tanaka C, Li LM, Fontes Baptista A, Hideki Okano A. Beyond the target area: an integrative view of tDCS-induced motor cortex modulation in patients and athletes. Journal of NeuroEngineering and Rehabilitation. 2019 Nov 15;16(1). Available from: http://dx.doi.org/10.1186/s12984-019-0581-1
Download PDF published in Journal of NeuroEngineering and Rehabilitation — DOI
Abstract
Transcranial Direct Current Stimulation (tDCS) is a non-invasive technique used to modulate neural tissue. Neuromodulation apparently improves cognitive functions in several neurologic diseases treatment and sports performance. In this study, we present a comprehensive, integrative review of tDCS for motor rehabilitation and motor learning in healthy individuals, athletes and multiple neurologic and neuropsychiatric conditions. We also report on neuromodulation mechanisms, main applications, current knowledge including areas such as language, embodied cognition, functional and social aspects, and future directions. We present the use and perspectives of new developments in tDCS technology, namely high-definition tDCS (HD-tDCS) which promises to overcome one of the main tDCS limitation (i.e., low focality) and its application for neurological disease, pain relief, and motor learning/rehabilitation. Finally, we provided information regarding the Transcutaneous Spinal Direct Current Stimulation (tsDCS) in clinical applications, Cerebellar tDCS (ctDCS) and its influence on motor learning, and TMS combined with electroencephalography (EEG) as a tool to evaluate tDCS effects on brain function.
Prof. Marom Bikson speaks at the Symposium on Manipulating Brain States at the Del Monte Institute for Neuroscience at the University of Rochester Medicine on ""The targeting limits of transcranial electrical stimulation"
Download slides