New Publication: Non-invasive brain stimulation for fatigue in PASC
Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC) . Brain Stimulation. 16 (203) 100-107
Kelly Santana a , Eduardo França a , Joao Sato ~ b , Ana Silva a , Maria Queiroz a , Julia de Farias a , Danniely Rodrigues a , Iara Souza a , Vanessa Ribeiro c , Egas Caparelli-Daquer d , Antonio L. Teixeira e, f , Leigh Charvet g , Abhishek Datta h, i , Marom Bikson h , Suellen Andrade a,
a Federal University of Paraíba, Joao Pessoa, Brazil ~ b Center of Mathematics, Computing and Cognition, Federal University of ABC, Santo Andre, Brazil c Department of Health, Government of Paraíba, Joao Pessoa, Brazil ~ d Nervous System Electric Stimulation Lab, Rio de Janeiro State University, Rio de Janeiro, Brazil e Department of Psychiatry and Behavioral Sciences, McGovern Medical School, University of Texas Health Science Center, Houston, United States f Faculdade Santa Casa BH, Belo Horizonte, Brazil g Department of Neurology, New York University Langone Health, New York, United States h Department of Biomedical Engineering, The City College of New York of CUNY, New York, United States i Research & Development, Soterix Medical, Inc., New York, United States
Abstract
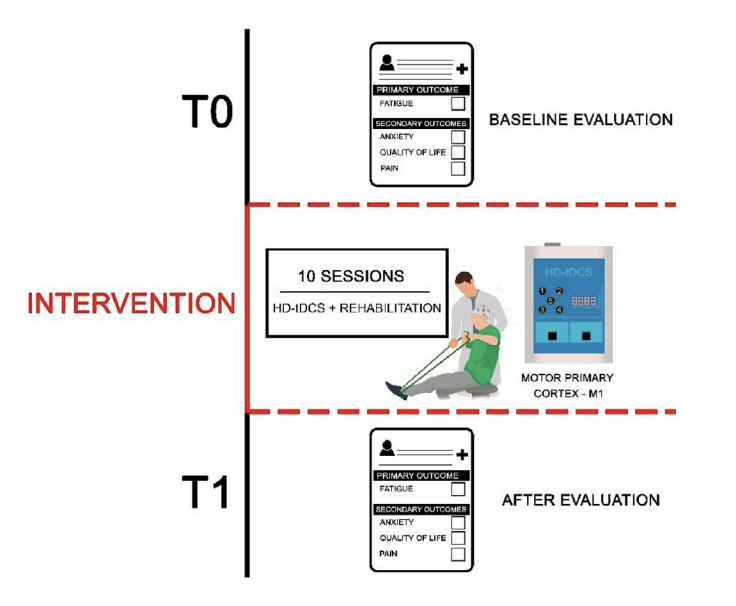
Background: and purpose: Fatigue is among the most common persistent symptoms following postacute sequelae of Sars-COV-2 infection (PASC). The current study investigated the potential therapeutic effects of High-Definition transcranial Direct Current Stimulation (HD-tDCS) associated with rehabilitation program for the management of PASC-related fatigue. Methods: Seventy patients with PASC-related fatigue were randomized to receive 3 mA or sham HD-tDCS targeting the left primary motor cortex (M1) for 30 min paired with a rehabilitation program. Each patient underwent 10 sessions (2 sessions/week) over five weeks. Fatigue was measured as the primary outcome before and after the intervention using the Modified Fatigue Impact Scale (MFIS). Pain level, anxiety severity and quality of life were secondary outcomes assessed, respectively, through the McGill Questionnaire, Hamilton Anxiety Rating Scale (HAM-A) and WHOQOL. Results: Active HD-tDCS resulted in significantly greater reduction in fatigue compared to sham HD-tDCS (mean group MFIS reduction of 22.11 points vs 10.34 points). Distinct effects of HD-tDCS were observed in fatigue domains with greater effect on cognitive (mean group difference 8.29 points; effect size 1.1; 95% CI 3.56e13.01; P < .0001) and psychosocial domains (mean group difference 2.37 points; effect size 1.2; 95% CI 1.34e3.40; P < .0001), with no significant difference between the groups in the physical subscale (mean group difference 0.71 points; effect size 0.1; 95% CI 4.47e5.90; P ¼ .09). Compared to sham, the active HD-tDCS group also had a significant reduction in anxiety (mean group difference 4.88; effect size 0.9; 95% CI 1.93e7.84; P < .0001) and improvement in quality of life (mean group difference 14.80; effect size 0.7; 95% CI 7.87e21.73; P < .0001). There was no significant difference in pain (mean group difference 0.74; no effect size; 95% CI 3.66e5.14; P ¼ .09). Conclusion: An intervention with M1 targeted HD-tDCS paired with a rehabilitation program was effective in reducing fatigue and anxiety, while improving quality of life in people with PASC.