Oct 18, 2023, Prof. Marom Bikson gives (via zoom) the Louisiana State University at Baton Rouge psychiatry grand rounds on ““A simple introduction to how neuromodulation devices work.”
Download slides PDF
Oct 18, 2023, Prof. Marom Bikson gives (via zoom) the Louisiana State University at Baton Rouge psychiatry grand rounds on ““A simple introduction to how neuromodulation devices work.”
Download slides PDF

Prof. Marom Bikson explains, with minimal technical language, How Neuromodulation For Pain Works.
Download SLIDES pdf
The lecture answers: What is "dose" in neuromodulation? How do neuromodulation devices control dose / what are "dose instructions"? When therapies work, what is it that is proven to work? What is the role of mechanisms in the invention of neuromodulation therapies?
What is the special role of the Gate Control Theory of Pain (by Melzack and Wall) and how did it drive modern neuromodulation for pain?
What is Transcutaneous Electrical Nerve Stimulation (TENS)? What is Peripheral Nerve Stimulation (PNS)? What is Spinal Cord Stimulation (SCS)? Dorsal Root Ganglion Stimulation (DRGs)? What are Evoked Compound Action Potentials (eCAPS)? What are Evoked Synaptic Activity Potentials (eSAPS)?
The lecture uses pain as the example but the broader concepts apply to all forms of neuromodulation / brain-stimulation.
Links to the cited works.
1. Fundamentals of Transcranial Electric and Magnetic Stimulation Dose: Definition, Selection, and Reporting Practices: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3346863/
2. Pain Mechanisms : A New Theory https://www.science.org/doi/10.1126/science.150.3699.971
3. Novel Evoked Synaptic Activity Potentials (ESAPs) Elicited by Spinal Cord Stimulation: https://www.eneuro.org/content/10/5/ENEURO.0429-22.2023.long
Citation: Bremner JD, Gazi AH, Lambert TP, Nawar A, Harrison AB, et al. Noninvasive Vagal Nerve Stimulation for Opioid Use Disorder. Ann Depress Anxiety. 2023; 10(1): 1117. PDF
Noninvasive Vagal Nerve Stimulation for Opioid Use Disorder
J Douglas Bremner, MD1,2,3; Asim H Gazi, BS4; Tamara P Lambert, MPH, MEng5; Afra Nawar, BS4; Anna B Harrison, MS4; Justine W Welsh, MD1; Viola Vaccarino, MD, PhD6,7; Kevin M Walton, PhD8; Nora Jaquemet, BS1; Kellen Mermin-Bunnell, BS1; Hewitt Mesfin, BS1; Trinity A Gray, BS1; Keyatta Ross, BS1; Georgia Saks BS5; Nikolina Tomic, MS4; Danner Affadzi, BS1; Marom Bikson, PhD9; Amit J Shah, MD, MSCR3,6,7; Kelly E Dunn, PhD10; Nicholas A Giordano, PhD, RN11; Omer T Inan, PhD4,5
1 Department of Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta GA
2 Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta GA
3 Atlanta Veterans Affairs Healthcare System, Decatur GA 4School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA
5 Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA
6 Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA
7 Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta GA
8 Clinical Research Grants Branch, Division of Therapeu- tics and Medical Consequences, National Institute on Drug Abuse, Bethesda, MD
9 Department of Biomedical Engineering, The City College of New York, New York, NY
10 Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore MD
11 Nell Hodgson Woodruff School of Nursing, Emory Uni- versity, Atlanta, GA
Abstract
Background: Opioid Use Disorder (OUD) is an escalating public health problem with over 100,000 drug overdose-related deaths last year most of them related to opioid overdose, yet treatment options remain limited. Non-invasive Vagal Nerve Stimulation (nVNS) can be delivered via the ear or the neck and is a non-medi- cation alternative to treatment of opioid withdrawal and OUD with potentially widespread applications.
Methods: This paper reviews the neurobiology of opioid with- drawal and OUD and the emerging literature of nVNS for the ap- plication of OUD. Literature databases for Pubmed, Psychinfo, and Medline were queried for these topics for 1982-present.
Results: Opioid withdrawal in the context of OUD is associated with activation of peripheral sympathetic and inflammatory sys- tems as well as alterations in central brain regions including ante- rior cingulate, basal ganglia, and amygdala. NVNS has the potential to reduce sympathetic and inflammatory activation and counter the effects of opioid withdrawal in initial pilot studies. Preliminary studies show that it is potentially effective at acting through sym- pathetic pathways to reduce the effects of opioid withdrawal, in addition to reducing pain and distress.
Conclusions: NVNS shows promise as a non-medication ap- proach to OUD, both in terms of its known effect on neurobiology as well as pilot data showing a reduction in withdrawal symptoms as well as physiological manifestations of opioid withdrawal.

Giuseppina Pilloni, Hyein Cho, Tian Esme Tian, Joerg Beringer, Marom Bikson, Leigh Charvet. (2023) . Immediate and Differential Response to Emotional Stimuli Associated With Transcranial Direct Current Stimulation for Depression: A Visual-Search Task Pilot Study.
Neuromodulation. https://doi.org/10.1016/j.neurom.2023.07.006 in press

Carine El Jamal, Ashley Harrie, Annalise Rahman-Filipiak, Alexandru D Iordan, Alexandre F DaSilva, Robert Ploutz-Snyder, Lara Khadr, Michael Vesia, Marom Bikson, Benjamin M Hampstead. (2023)
Tolerability and blinding of high-definition transcranial direct current stimulation among older adults at intensities of up to 4 mA per electrode.
Brain Stimulation. https://doi.org/10.1016/j.brs.2023.08.025 in press
Marom Bikson organizes the Neuromodulation The Science (NTS) component of the 30th Napa Pain Conference, in Napa, California, on August 18-19, 2023. Program and details.
Dr. Bikson will lecture on “How Neuromodulation for Pain Works.” . Download talk slides

Dr. Marom Bikson lectures on “Neuro-vascular modulation: what a new mechanism suggests about how brain stimulation works and how to interpret hemodynamic imaging?” at the 3rd Annual Brain & Human Body Modeling (BHBM) Conference
(Online format with in-person participation) August 17-18, 2023
Slides PDF

Soleimani, G., Nitsche, M. A., Bergmann, T. O., Towhidkhah, F., Violante, I. R., Lorenz, R., Kuplicki, R., Tsuchiyagaito, A., Mulyana, B., Mayeli, A., Ghobadi-Azbari, P., Mosayebi-Samani, M., Zilverstand, A., Paulus, M. P., Bikson, M., & Ekhtiari, H. (2023). Closing the loop between brain and electrical stimulation: towards precision neuromodulation treatments. Translational Psychiatry, 13, 279(2023). https://doi.org/10.1038/s41398-023-02565-5 PDF

PAPER
Niranjan Khadka, Cynthia Poon, Limary M Cancel, John M Tarbell and Marom Bikson
Published 24 July 2023
Journal of Neural Engineering, Volume 20, Number 4 Citation Niranjan Khadka et al 2023 J. Neural Eng. 20 046014DOI 10.1088/1741-2552/ace4f4
Paper PDF
Objective. Transcranial direct current stimulation (tDCS) generates sustained electric fields in the brain, that may be amplified when crossing capillary walls (across blood-brain barrier, BBB). Electric fields across the BBB may generate fluid flow by electroosmosis. We consider that tDCS may thus enhance interstitial fluid flow. Approach. We developed a modeling pipeline novel in both (1) spanning the mm (head), μm (capillary network), and then nm (down to BBB tight junction (TJ)) scales; and (2) coupling electric current flow to fluid current flow across these scales. Electroosmotic coupling was parametrized based on prior measures of fluid flow across isolated BBB layers. Electric field amplification across the BBB in a realistic capillary network was converted to volumetric fluid exchange. Main results. The ultrastructure of the BBB results in peak electric fields (per mA of applied current) of 32–63 across capillary wall and >1150 in TJs (contrasted with 0.3 in parenchyma). Based on an electroosmotic coupling of 1.0 × 10−9 – 5.6 × 10−10 per , peak water fluxes across the BBB are 2.44 × 10−10 – 6.94 × 10−10, with a peak 1.5 × 10−4 – 5.6 × 10−4 interstitial water exchange (per mA). Significance. Using this pipeline, the fluid exchange rate per each brain voxel can be predicted for any tDCS dose (electrode montage, current) or anatomy. Under experimentally constrained tissue properties, we predicted tDCS produces a fluid exchange rate comparable to endogenous flow, so doubling fluid exchange with further local flow rate hot spots ('jets'). The validation and implication of such tDCS brain 'flushing' is important to establish.

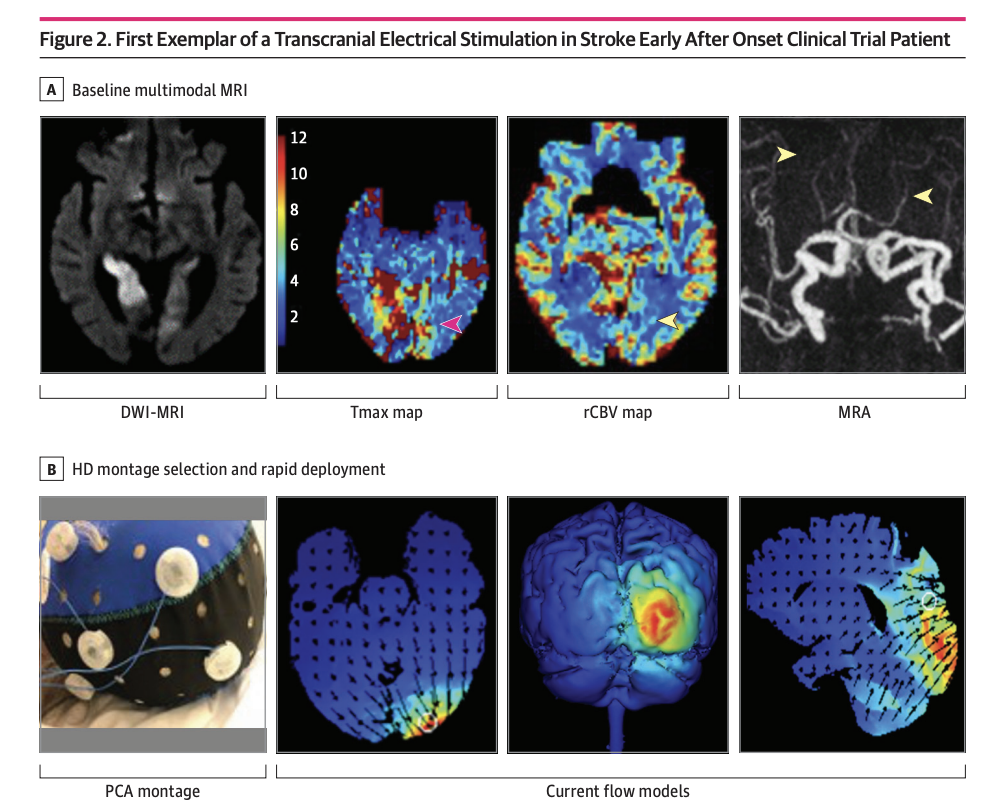
Clinical Trial JAMA Network Open. 2023 Jun 1;6(6):e2319231. doi: 10.1001/jamanetworkopen.2023.19231.
High-definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial
Mersedeh Bahr-Hosseini, Kambiz Nael, Gozde Unal, Marco Iacoboni, David S Liebeskind, Marom Bikson, Jeffrey L Saver; TESSERACT Trial Group
PMID: 37342040 PMCID: PMC10285579 DOI: 10.1001/jamanetworkopen.2023.19231
Key Points
Question Is application of high-definition cathodal transcranial direct current stimulation (HD C-tDCS) as a noninvasive targeted acute ischemic stroke treatment strategy feasible and well-tolerated, and does it show signals of beneficial effects?
Findings In this randomized clinical trial enrolling 10 patients (7 active, 3 sham), HD C-tDCS was started within a median 12.5 minutes of randomization in final enrolled patients and showed good tolerability with signals of favorable effects on salvage of threatened tissue.
Meaning These results suggest that HD C-tDCS is a noninvasive targeted acute ischemic stroke treatment strategy that can be efficiently applied in emergency settings and warrants testing in larger multicenter trials.
Abstract
Importance Cathodal transcranial direct current stimulation (C-tDCS) provides neuroprotection in preclinical models of acute ischemic stroke (AIS) by inhibiting peri-infarct excitotoxic effects and enhancing collateral perfusion due to its vasodilatory properties.
Objective To report the first-in-human pilot study using individualized high-definition (HD) C-tDCS as a treatment of AIS.
Design, Setting, and Participants This randomized clinical trial was sham controlled with 3 + 3 dose escalation design, and was conducted at a single center from October 2018 to July 2021. Eligible participants were treated for AIS within 24 hours from onset, had imaging evidence of cortical ischemia with salvageable penumbra, and were ineligible for reperfusion therapies. HD C-tDCS electrode montage was selected for each patient to deliver the electric current to the ischemic region only. Patients were followed for 90 days.
Main Outcomes and Measures Primary outcomes were feasibility, assessed as time from randomization to study stimulation initiation; tolerability, assessed by rate of patients completing the full study stimulation period; and safety, assessed by rates of symptomatic intracranial hemorrhage at 24 hours. The efficacy imaging biomarkers of neuroprotection and collateral enhancement were explored.
Results A total of 10 patients with AIS were enrolled, 7 were randomized to active treatment and 3 to sham. Patient age was mean (SD) 75 (10) years old, 6 (60%) were female, and National Institutes of Health Stroke Scale score was mean (SD) 8 (7). Two doses of HD C-tDCS (1 milliamp [mA] for 20 minutes and 2 mA for 20 minutes) were studied. The speed of HD C-tDCS implementation was a median (IQR) 12.5 minutes (9-15 minutes) in the last 4 patients. Patients tolerated the HD C-tDCS with no permanent stimulation cessation. The hypoperfused region was reduced by a median (IQR) 100% (46% to 100%) in the active group vs increased by 325% (112% to 412%) in sham. Change in quantitative relative cerebral blood volume early poststimulation was a median (IQR) 64% (40% to 110%) in active vs −4% (−7% to 1%) sham patients and followed a dose-response pattern. Penumbral salvage in the active C-tDCS group was median (IQR) 66% (29% to 80.5%) vs 0% (IQR 0% to 0%) in sham.
Conclusion and Relevance In this randomized, first-in-human clinical trial, HD C-tDCS was started efficiently and well tolerated in emergency settings, with signals of beneficial effect upon penumbral salvage. These results support advancing HD C-tDCS to larger trials.
Trial Registration ClinicalTrials.gov Identifier: NCT03574038
Nigel Gebodh, PhD Candidate mentored by Professor Marom Bikson has recently been highlighted by the NIH-funded Graduate Research Training Initiative for Student Enhancement (G-RISE) Program. Learn more about Nigel and his research by watching his video below!
On Friday, June 2, 2023, The Neural Engineering Lab partook in CCNY’s Commencement, celebrating with four doctoral students who defended in Fall 2023 or Spring 2023, or will defend in Summer 2023. Congratulations!
Mentor: Marom Bikson, PhD
Graduate: Adantchede Louis Zannou, PhD
Mentor: Lucas C. Parra, PhD
Graduates: Lukas Hirsch, PhD; Maximilian Nentwich, PhD; and Forouzan Vasheghani Farahani, PhD

Neural Engineering Lab Doctoral Graduates: Maximilian Nentwich, Forouzan Vasheghani Farahani, Lukas Hirsch, Adantchede Louis Zannou

Adantchede Louis Zannou with his PhD mentor, Professor Marom Bikson

Forouzan Vasheghani Farahani, Maximilian Nentwich, and Lukas Hirsch with their PhD mentor, Professor Lucas Parra
See the graduates be “hooded” in the link above!
2:42:47 to 2:44:45
eNeuro 2023 Apr 27;ENEURO.0429-22.2023. doi: 10.1523/ENEURO.0429-22.2023.
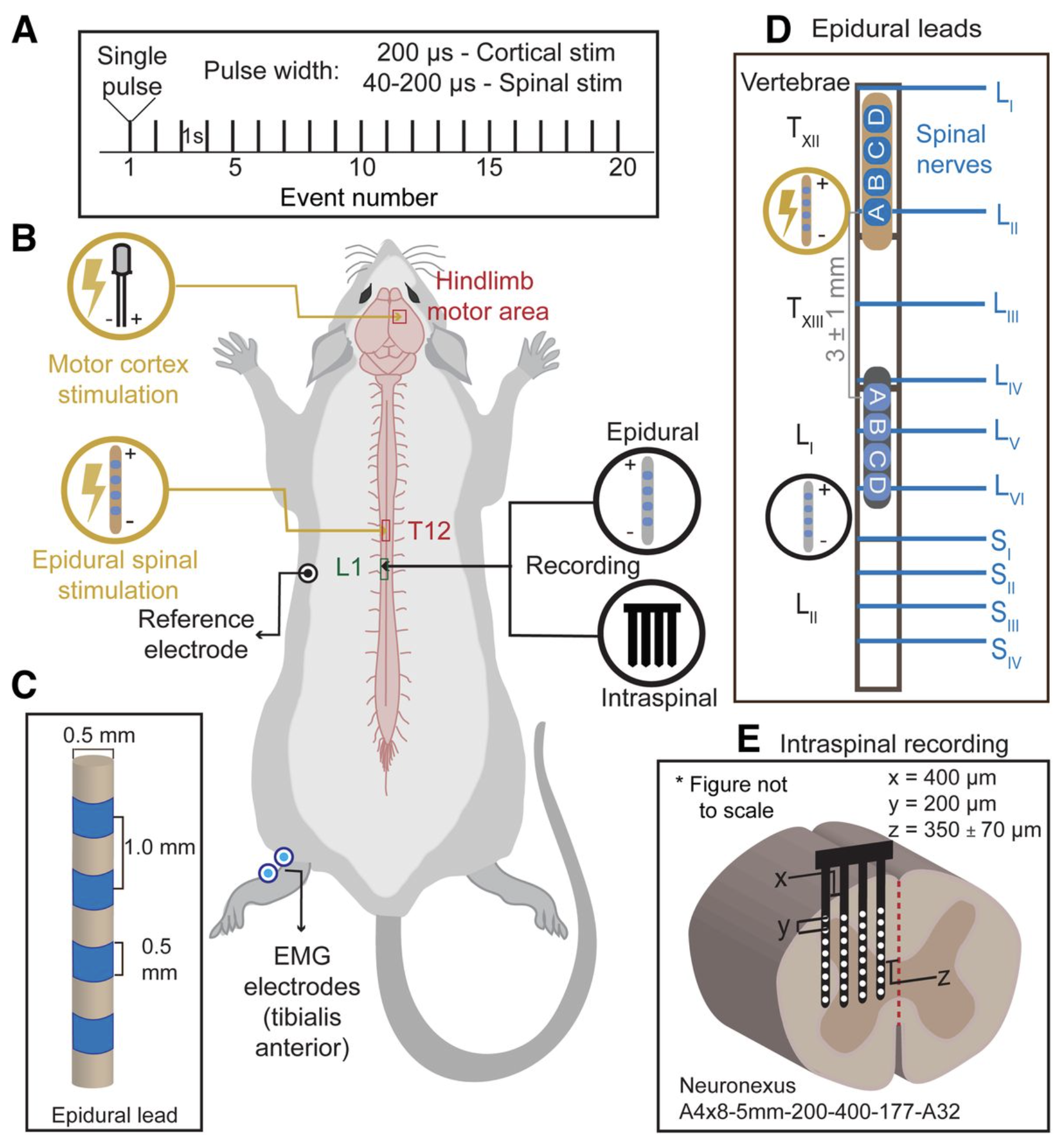
Novel Evoked Synaptic Activity Potentials (ESAPs) elicited by Spinal Cord Stimulation
Mahima Sharma 1, Vividha Bhaskar 2, Lillian Yang 3, Mohamad FallahRad 2, Nigel Gebodh 2, Tianhe Zhang 4, Rosana Esteller 4, John Martin 3, Marom Bikson 2
PMID: 37130780 DOI: 10.1523/ENEURO.0429-22.2023
Abstract: Spinal cord stimulation (SCS) evokes fast epidural Evoked Compound Action Potential (ECAPs) that represent activity of dorsal column axons, but not necessarily a spinal circuit response. Using a multimodal approach, we identified and characterized a delayed and slower potential evoked by SCS that reflects synaptic activity within the spinal cord. Anesthetized female Sprague Dawley rats were implanted with an epidural SCS lead, epidural motor cortex stimulation electrodes, an epidural spinal cord recording lead, an intraspinal penetrating recording electrode array, and intramuscular electromyography (EMG) electrodes in the hindlimb and trunk. We stimulated the motor cortex or the epidural spinal cord and recorded epidural, intraspinal, and EMG responses. SCS pulses produced characteristic propagating ECAPs (composed of P1, N1, and P2 waves with latencies <2 ms) and an additional wave ("S1") starting after the N2. We verified the S1-wave was not a stimulation artifact and was not a reflection of hindlimb/trunk EMG. The S1-wave has a distinct stimulation-intensity dose response and spatial profile compared to ECAPs. CNQX (a selective competitive antagonist of AMPA receptors) significantly diminished the S1-wave, but not ECAPs. Furthermore, cortical stimulation, which did not evoke ECAPs, produced epidurally detectable and CNQX-sensitive responses at the same spinal sites, confirming epidural recording of an evoked synaptic response. Finally, applying 50 Hz SCS resulted in dampening of S1-wave, but not ECAPs. Therefore, we hypothesize that the S1-wave is synaptic in origin, and we term the S1-wave type responses: Evoked Synaptic Activity Potentials (ESAPs). The identification and characterization of epidurally recorded ESAPs from the dorsal horn may elucidate SCS mechanisms.Significance StatementSpinal cord stimulation (SCS) is an established treatment for chronic pain and has applications to other disorders and neurorehabilitation. Notwithstanding decades of trials and research, questions remain about SCS mechanisms of action - and indicators thereof. Recent technological developments have enabled the detection of Evoked Compound Action Potential (ECAPs) - reflecting synchronous activity of the dorsal column axons activated by SCS. However, ECAP is not a direct measure of sensory processing in the dorsal horn. Here, we identify and characterize a novel electrophysiological signal that is evoked and detectable by epidural SCS electrodes and reflects spinal synaptic currents. This new signal, termed an Evoked Synaptic Activity Potential (ESAP), is thus a novel means with which to interrogate spinal gray matter circuits during SCS.
Keywords: EMG; Epidural recording; Evoked Compound Action Potential (ECAP); Evoked Synaptic Activity Potentials (ESAP).
Marom Bikson, Ana Ganho-Ávila, Abhishek Datta, Bernadette Gillick, Morten Goertz Joensson, Sungjin Kim, Jinuk Kim, Adam Kirton, Kiwon Lee, Timothy Marjenin, Balder Onarheim, Erik M. Rehn, Alexander T. Sack, Gozde Unal.
Limited output transcranial electrical stimulation 2023 (LOTES-2023): Updates on engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk,
Brain Stimulation, 2023, ISSN 1935-861X,
https://doi.org/10.1016/j.brs.2023.05.008. PDF
Abstract: The objective and scope of this Limited Output Transcranial Electrical Stimulation 2023 (LOTES-2023) guidance is to update the previous LOTES-2017 guidance. These documents should therefore be considered together. The LOTES provides a clearly articulated and transparent framework for the design of devices providing limited output (specified low-intensity range) transcranial electrical stimulation for a variety of intended uses. These guidelines can inform trial design and regulatory decisions, but most directly inform manufacturer activities - and hence were presented in LOTES-2017 as “Voluntary industry standard for compliance controlled limited output tES devices”. In LOTES-2023 we emphasize that these standards are largely aligned across international standards and national regulations (including those in USA, EU, and South Korea), and so might be better understood as “Industry standards for compliance controlled limited output tES devices”. LOTES-2023 is therefore updated to reflect a consensus among emerging international standards, as well as best available scientific evidence. “Warnings” and “Precautions” are updated to align with current biomedical evidence and applications. LOTES standards applied to a constrained device dose range, but within this dose range and for different use-cases, manufacturers are responsible to conduct device-specific risk management.
Annals of Biomedical Engineering. 2023. DOI: 10.1007/s10439-023-03211-3
Non-invasive electrical brain stimulation optimized for targeted and safe BBB openingNeeraj Raghuraman Rajagopalan, William-Ray Vista, Masashi Fujimori, Laurien G. P. H. Vroomen, Juan M. Jiménez, Niranjan Khadka, Marom Bikson, Govindarajan Srimathveeravalli
Abstract
High-voltage pulsed electric felds (HV-PEF) delivered with invasive needle electrodes for electroporation applications is known to induce of-target blood–brain barrier (BBB) disruption. In this study, we sought to determine the feasibility of minimally invasive PEF application to produce BBB disruption in rat brain and identify the putative mechanisms mediating the efect. We observed dose-dependent presence of Evans Blue (EB) dye in rat brain when PEF were delivered with a skull mounted electrode used for neurostimulation application. Maximum region of dye uptake was observed while using 1500 V, 100 pulses, 100 µs and 10 Hz. Results of computational models suggested that the region of BBB disruption was occurring at thresholds of 63 V/cm or higher; well below intensity levels for electroporation. In vitro experiments recapitulating this efect with human umbilical vein endothelial cells (HUVEC) demonstrated cellular alterations that underlie BBB manifests at low-voltage high-pulse conditions without afecting cell viability or proliferation. Morphological changes in HUVECs due to PEF were accompanied by disruption of actin cytoskeleton, loss of tight junction protein—ZO-1 and VE-Cadherin at cell junctions and partial translocation into the cytoplasm. Uptake of propidium iodide (PI) in PEF treated conditions is less than 1% and 2.5% of total number of cells in high voltage (HV) and low-voltage (LV) groups, respectively, implying that BBB disruption to be independent of electroporation under these conditions. 3-D microfabricated blood vessel permeability was found to increase signifcantly following PEF treatment and confrmed with correlative cytoskeletal changes and loss of tight junction proteins. Finally, we show that the rat brain model can be scaled to human brains with a similar efect on BBB disruption characterized by electric feld strength (EFS) threshold and using a combination of two bilateral HD electrode configurations.

Prof. Marom Bikson gives an invited talk at the 2023 Northeast Bioengineering Conference on March 31, 2023 in Drexel University, Philadelphia, PA. Conference detail.
Dr. Bikson’s lecture is “Functional Neuroimaging as the Key to Effective Neurostimulation: Neuro-vascular Modulation?” Download slides PDF
Thanks to event co-host Dr. Hasan Ayaz for these photos from talk:

Slides from Prof. Marom Bikson’s guest lecture on Transcranial Electrical Stimulation (and non-invasive cranial nerves stimulation) to Dr. Bin He’s Neural Engineering course at Carnegie Mellon University on March 20, 2023.
Prof. Marom Bikson will 2023 Bioelectronic Medicine Forum (April 4, 2023) held at New York Academy of Medicine, New York, NY (and Zoom). More program details
Download Pro. Bikson’s presentation on “Personalized medicine (Neuromodulation technology) has individually tuned dose. “. PDF
Tentative Agenda, All Times EDT
8:30-8:45
Welcome and Introductions
8:45-9:00
Overview of the Bioelectronic Medicine Industry
James Cavuoto, Editor, Neurotech Reports
BioElectRx Business Report Editor James Cavuoto presents an overview of the bioelectronic medicine industry, including key players, technological roots, and market projections.
9:00-9:30
Keynote Address
Helen Mayberg, M.D., Director, Center for Advanced Circuit Therapeutics, Icahn School of Medicine at Mount Sinai
One of the pioneers in the field of neuromodulation for psychiatric disorders discusses the interplay between pharmacological and device interventions in treating disease.
9:30-10:15
Investment in Bioelectronic Medicine
JoJo Platt, Senior Contributing Editor, Neurotech Reports, Moderator
Josh Schulman, Ph.D., Chief Science Officer, Joy Ventures
Amy Kruse, Ph.D., Chief Innovation Officer, Satori Capital
In this session, investment professionals active in the life sciences industry will offer their views on the investment climate for bioelectronic medicine.
10:15-10:45
Refreshment Break
10:45-11:30
Entrepreneur Panel I
James Cavuoto, Editor, Neurotech Reports, Moderator
Brad Schmidt, Ph.D., CEO, Panaxium
Sandro Ferrari, Ph.D., Director of Operations, WISE srl
Presentations from executives of emerging bioelectronic medicine firms.
11:30-12:00
Exploiting the Enteric Nervous System
James Cavuoto, Editor, Neurotech Reports, Moderator
Victor Pikov, Ph.D., CEO, Medipace Inc.
Jennifer French, Executive Director, Neurotech Network
The brain-gut connection not only opens the door to new bioelectronic medicine therapies for gastrointestinal disorders, it also offers a potential back door to the CNS. In this session, we'll explore potential new therapies and opportunities to collaborate with biopharma firms.
12:00-1:00
Luncheon
1:00-1:30
Luncheon Speaker
Will Pitkin, Senior Director of Business Development and Neuromodulation Strategy, Cirtec Medical
1:30-2:15
Biomarkers Close the Loop in Bioelectronic Medicine
Victor Pikov, Ph.D., Contributing Editor, Neurotech Reports, Moderator
Marom Bikson, Ph.D., Professor of Biomedical Engineering, City College of New York
Imanuel Lerman, M.D., Founder, InflammaSense
The ability to track neural activity offers bioelectronic medicine vendors several advantages. In this session, we'll look at examples of closed-loop neuromodulation and discuss new areas that stand to beneift.
2:15-3:00
Entrepreneur Panel II
James Cavuoto, Editor, Neurotech Reports, Moderator
Marc Powell, Ph.D., CEO, Reach Neuro Inc.
Karen Crow, CEO and Co-Founder, NeuroGeneces
Presentations from executives of emerging bioelectronic medicine firms.
3:00-3:30
Refreshment Break
3:30-4:15
Entrepreneur Panel III
James Cavuoto, Editor, Neurotech Reports, Moderator
Christine Aytug, Director of Business Development, Evren Technologies
Jacob Robinson, Ph.D., Co-Founder and CEO, Motif Neurotech
Presentations from executives of emerging bioelectronic medicine firms.
4:15-5:00
Who Foots the Bill? New Economic Models and Reimbursement Options
Jeremy Koff, Senior Consulting Editor, Neurotech Reports, Moderator
Renee Ryan, CEO, Cala Health
Mark Domyahn, Partner, J.D. Lymon Group
Bioelectronic medicine therapies occupy some new terrain when it comes to payment models and reimbursement strategies. In this session, we'll explore recent trends in device coverage and look at best practices for obtaining reimbursement.
5:00-6:30
Cocktail Reception
(Update): The articles was featured on the cover of the issue of Brain Stimulation
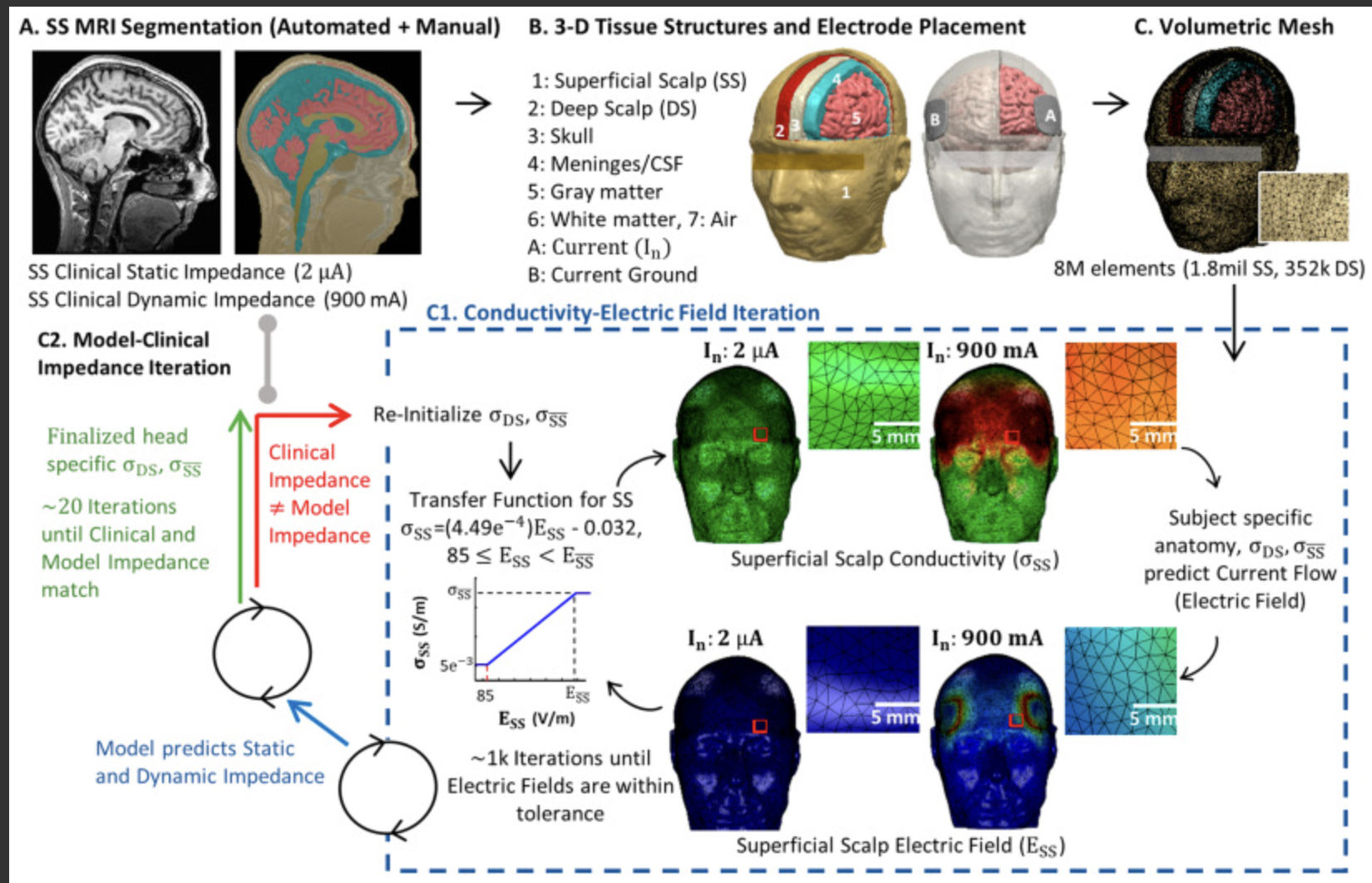
Unal, G., Poon, C, FallahRad, M., Thahsin, M. Argyelan, M., Bikson, M. Quasi-static pipeline in electroconvulsive therapy computational modeling. Brain Stimulation. DOI: https://doi.org/10.1016/j.brs.2023.03.007 PDF
__________________________________
Why it matters:
As in all neuromodulation domains, computational current flow models of ECT are increasingly used to inform device/therapy optimization and study mechanisms of actions. ECT is unique in applying repeated high-intensity electrical pulse across the skin. The quasi-static assumption is always applied in such models.
But we believe the skin responds in a complex (frequency-specific, intensity sensitive, non-linear) way to the ECT stimulation, which makes the application of the quasi-static assumption problematic.
In this paper, we don't ignore this problem, but address it head on.
Good news: we develop a solution that allows a quasi-static pipeline to be used, under a central (experimentally justified) assumption of a 'representative-frequency'. This "rescues" past ECT modeling efforts, and allows for more realistic consideration of how static-impedance and dynamic-impedance arise and can inform ECT analysis.
__________________________________
ABSTRACT
Computational models of current flow during Electroconvulsive Therapy (ECT) rely on the quasi-static assumption, yet tissue impedance during ECT may be frequency specific and change adaptively to local electric field intensity.
We systematically consider the application of the quasi-static pipeline to ECT under conditions where 1) static impedance is measured before ECT and 2) during ECT when dynamic impedance is measured. We propose an update to ECT modeling accounting for frequency-dependent impedance.
The frequency content on an ECT device output is analyzed. The ECT electrode-body impedance under low-current conditions is measured with an impedance analyzer. A framework for ECT modeling under quasi-static conditions based on a single device-specific frequency (e.g., 1 kHz) is proposed.
Impedance using ECT electrodes under low-current is frequency dependent and subject specific, and can be approximated at >100 Hz with a subject-specific lumped parameter circuit model but at <100 Hz increased non-linearly. The ECT device uses a 2 μA 800 Hz test signal and reports a static impedance that approximate 1 kHz impedance. Combined with prior evidence suggesting that conductivity does not vary significantly across ECT output frequencies at high-currents (800–900 mA), we update the adaptive pipeline for ECT modeling centered at 1 kHz frequency. Based on individual MRI and adaptive skin properties, models match static impedance (at 2 μA) and dynamic impedance (at 900 mA) of four ECT subjects.
By considering ECT modeling at a single representative frequency, ECT adaptive and non-adaptive modeling can be rationalized under a quasi-static pipeline.
Kevin Walsh shares research titled "Heart Rate Variability Measured via Non Invasive Brain Stimulation Devices, Tissue Impedance, and Deep Learning" in a poster presentation competition.
Follow his research here:
