Esmaeilpour Z, Milosevic M, Azevedo K, Khadka N, Navarro J, Brunoni A, Popovic MR, Bikson M, Fonoff ET
Download: PDF published in Brain Stimulation DOI
Abstract
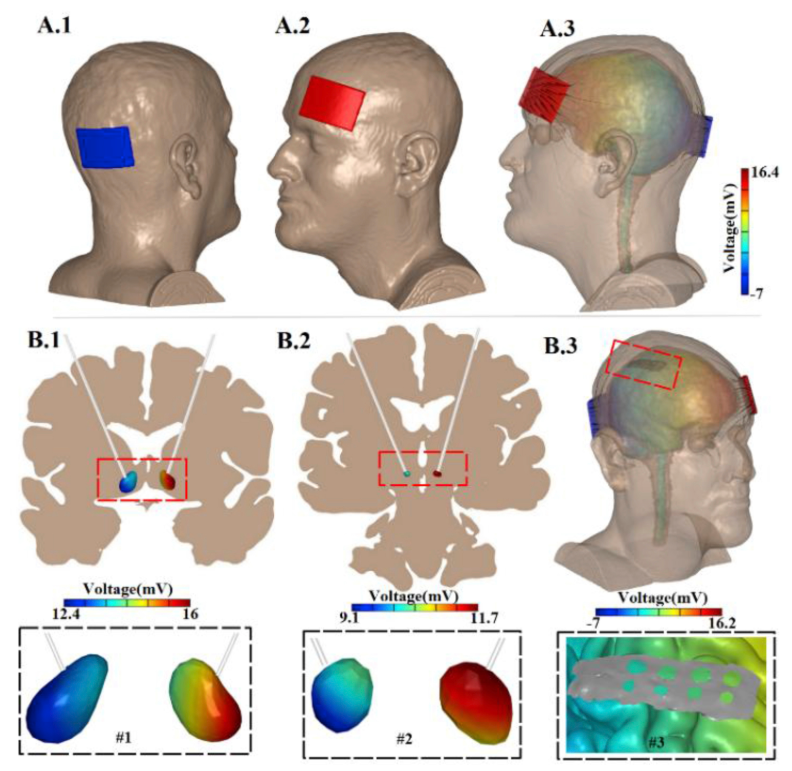
During transcranial direct current stimulation (tDCS) weak (1-2 mA) currents are applied across the head, producing low-intensity electric fields in the brain with the intention of modulating neuronal function. For any application of tDCS spanning cognitive neuroscience and neuropsychiatric therapies [1], understanding the amount of current delivered to the brain and the resulting electric field (in V/m) produced is thus important. In animal studies, direct current (DC) electric fields as low as 0.2-1.0 V/m influence neuronal excitability and plasticity [2, 3]. Since measurement of electric field in human is difficult to implement, high-resolution finite element head models [4] have been used to predict brain current flow during tDCS [5] – with many reports adapting a standard (S#) head [6-8]. There have been previous attempts to validate computational model predictions indirectly with scalp electrodes [9] and neurophysiology [10] during tDCS, as well as directly using intra-cranial electrodes, but not with DC stimulation [11, 12]. In this pilot study, DC voltage was measured using deep brain stimulation (DBS) and epidural lead electrodes during application of tDCS in human subjects. The results were evaluated against a standard (S#) head model. The model predictions of voltage produced across cortical (epidural) electrodes were consistent with recorded data, while subcortical (DBS) voltages were sensitive to conductivity assigned to subcortical structures.